
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
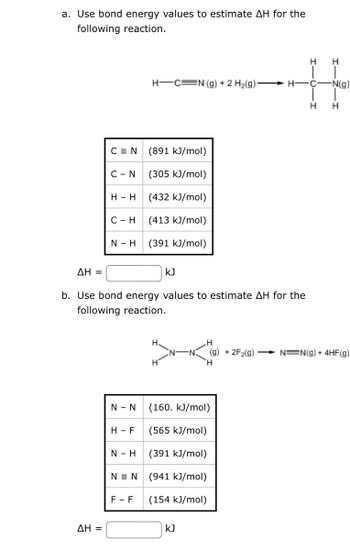
Transcribed Image Text:a. Use bond energy values to estimate AH for the
following reaction.
CEN
ΔΗ =
C - N
H - H
C-H
N - H
ΔΗ =
b. Use bond energy values to estimate AH for the
following reaction.
H-F
N - H
H-C=N (g) + 2 H₂(g):
NEN
(891 kJ/mol)
(305 kJ/mol)
(432 kJ/mol)
(413 kJ/mol)
(391 kJ/mol)
N - N (160. kJ/mol)
(565 kJ/mol)
(391 kJ/mol)
(941 kJ/mol)
(154 kJ/mol)
F-F
kJ
H-
kJ
H
C-
H
H
-N(g)
H
H
(g) +2F2(g) → N=N(g) + 4HF(g)
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction below, calculate the enthalpy change using the appropriate bond energies in the table on the next page. C3H8(g) + 5O2(g)à3CO2(g)+ 4H2O(g)arrow_forwardTrue or False: This reaction is endothermic because more energy is needed to break up the reactants versus the amount given off by the products. Use the reaction and bond information to answer the question. H-H +0=C = 0 - H-C = O | O-H H2 + CO, → CH,O. Reactant bond energies: H-H is 432 kJ/mol, C=O is 799 kJ/mol Product bond energies: C-H is 413 kJ/mol, C=O is 745 kJ/mol, C-O is 358 kJ/mol, O-H is 467 kJ/mol ○ True ○ False 1arrow_forward2. Use the table below to estimate AH for the reaction NO + O3 → NO2 + O2. Does this reaction consume or produce heat? Single Bond Energies (kJ/mol of bonds) н сN oS F C Br I Н 436 C 413 346 N 391 305 163 O 463 358 201 146 S 347 272 226 - F 565 485 283 190 284 155 Cl 432 339 192 218 255 253 242 Br 366 285 201 217 249 216 193 - I 299 213 201 278 208 175 151 - - Multiple Bond Energies (kJ/mol of bonds) C=C 602 C=N 615 C=O 799 C=C 835 CEN 887 C=O 1072 N=N 418 N=O 607 N=N 945 O=O 498arrow_forward
- A.) Which C–O bond has the smallest bond energy? CO32– CO2 CO B.) Which reaction is exothermic? 2CO(g) + O2(g) → 2CO2(g) CO2(g) → C(s) + O2(g) CO2(g) + H2O(l) → 2H+(aq) + CO32–(aq)arrow_forwardCalculate the DHf for HBr in kJ/(mol HBr) using the bond energies given below. H-H 436 kJ/mol Br-Br 190 kJ/mol H-Br 370 kJ/molarrow_forwardE C Bond energy = The enthalpy change for the following reaction is -137 kJ. Using bond energies, estimate the C-C bond energy in C₂H6 (9). C₂H4 (9) + H₂(g) → C₂H6 (g) Bond Bond Energy (kJ/mol) C=C H-H C-H $ 4 Submit Answer R F V % 5 Show Hint T G rences] Use the References to access important values if needed for this question. kJ/mol 602 436 413 Retry Entire Group 4 more group attempts remaining B Cengage Learning Cengage Technical Supportarrow_forward
- How much energy (in kJ) is required to separate (break) one mole of H-H bonds?arrow_forwardReferences] Consider the following reaction: H. H. CI CI CC (9) + CLg) H-C-C-H( AH-172 kJ H. H H Estimate the carbon-chlorine bond energy given that the C-0 bond energy is 347 /mol, the 0-C bond energy is 614 kJmol, and the Cl-O1 bond energy is 239 KJmol. Doa Umolarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY