
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
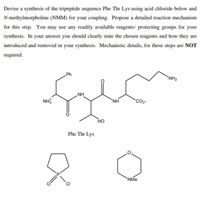
Transcribed Image Text:Devise a synthesis of the tripeptide sequence Phe Thr Lys using acid chloride below and
N-methylmorpholine (NMM) for your coupling. Propose a detailed reaction mechanism
for this step. You may use any readily available reagents/ protecting groups for your
synthesis. In your answer you should clearly state the chosen reagents and how they are
introduced and removed in your synthesis. Mechanistic details, for these steps are NOT
required.
Ph
`NH2
NH
NH
`NH
CO
HO
Phe Thr Lys
`NMe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show how you would synthesize 1-amino-2-methylbutane from 2-butanone and any other necesary reagentsarrow_forwardWhich of the following compounds will react with methylamine to form the imine shown below?arrow_forwardYou synthesize the following compound via a base-mediated synthesis. Upon workup, you find that the compound is soluble in the aqueous solvent from which the reaction was conducted. However, you need to vacuum filter the solid as indicated in the procedure. What do you still need to do before filtration? Add more base to the reaction, it must not be complete. Heat the reaction mixture to accelerate precipitation. O Do an acid wash. O Do a base wash.arrow_forward
- give the retrosynthetic analysis which has an aromatic starting compound that gives this product and then the forward reaction stating all the reagentsarrow_forwardGiven the target molecule and it's synthons, propose it's synthetic equivalents and a forward synthesis.arrow_forwardShow the retrosynthetic analysis and foreward reaction of the following compounds. Please draw out the structures too.arrow_forward
- Make an electron-flow-mechanism for this synthetic scheme. This involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shows. (BENZENE -> 2-ACETOXYBENZOIC ACID or ASPIRIN)arrow_forwardBy using only amide/Peptide bond forming rxn and a lkylation rxns. Provide all the reaction steps with the reagent needed and conditions with out showing the mechanism for each step to form the product. Note: Use protecting groups like (Boc, Fmoc, and others) H¸NNH rxns. By using only amide / Peptide bond forming rxn and alkylation Provide all the reaction steps with the reagent needed and conditions showing the mechanism for each step to form the product. protecting groups. like (Boc, Fmoc, and others) with out Note: Use HN H₂N. -NH₂- + ΝΗarrow_forwardGive a clear explanation handwritten answer..give the mechanism of given bleow reactionsarrow_forward
- The following reaction is an example of a "thiol/ene" Michael reaction. It's commonly used to modify polymers as shown below. Provide a mechanism. Triethylamine is used as a base.arrow_forwardافي can you create an original syntheic scheme to the given target and write a paragraph that descibe the reagents/solvents being used and the name of the mechanism/reaction perfomed (should be like a scientific journal article) C งarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY