
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
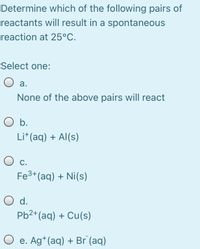
Transcribed Image Text:Determine which of the following pairs of
reactants will result in a spontaneous
reaction at 25°C.
Select one:
O a.
None of the above pairs will react
O b.
Li*(aq) + Al(s)
С.
Fe3+(aq) + Ni(s)
O d.
Pb2+(aq) + Cu(s)
O e. Ag*(aq) + Br (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15arrow_forwardDetermine which of the following pairs of reactants will result in a spontaneous reaction at 25°C. Zn(s) + Na+(aq) Sn4+(aq) + Zn(s) Ni(s) + Ba2+(aq) Cr3+(aq) + Sn(s) None of the above pairs will react.arrow_forwardConsider the reaction described by the chemical equation shown. 3 C,H,(g) → C,H,(1) AHan = -633.1 kJ Use the data from the table of thermodynamic properties to calculate the value of ASxn at 25.0 °C. ASPxn = J.K-!arrow_forward
- Question 6 Calculate the AG for the following process at 100 K and 300 K. Use the thermodynamic data from Appendix G in OpenStax Chemistry, 2e. If one exists, calculate the temperature (in K) at which the process switches between spontaneous and non-spontaneous. ZaCl() Zn (aq) + 2 Cl (ag) Report G to the tenths place. AG T= " K ப Parrow_forwardUsing the appendix informa=on in your textbook calculate E° from ΔG° for the following reaction:CH3OH(l) + 3/2O2(g) ➝ CO2(g) + 2H2O(l)As the temperature increases would you expect E to increase or decrease. Justify your response.arrow_forward3. If 20 g of KNO3 is dissolved in 50 mL of water at 4 °C, is the system in equilibrium? If not, what spontaneous process will occur? What is the value of AG (NOT AG) for the system, as prepared, before any reaction occurs? KNO3, K², NO₂ (44) NO3(aq) AH Rarrow_forward
- Determine which of the following pairs of reactants will result in a spontaneous reaction at 25°C. Zn(s) + Na+(aq) Sn4+(aq) + Zn(s) Ni(s) + Ba2+(aq) Cr3+(aq) + Sn(s) None of the above pairs will react.arrow_forwardTrue or false? Why?arrow_forwardWhat is the equilibrium constant at 25.00°C for the reaction below? Pb(s) + 2 NiO(s) → PbO2(s) + 2 Ni(s) ΔG° = 206.1 kJ/molarrow_forward
- A Moving to another question will save this response. Question 13 Under standard conditions, a positive value of AG° for a reaction indicates that: O the reaction is at equilibrium O the reaction is non-spontaneous in the forward direction O the reaction favors formation of products O the reaction is spontaneous in the forward direction O the reaction cannot reach equilibrium A Moving to another question will save this response. MacBook Air esc F2 F3 F4 F5 F1 @ #3 %$4 7 3 4. 6 W R. Breathe easy.arrow_forwardDescribe each reaction as spontaneous or non-spontaneous. Au^3+ + Fe^3+ -----> Fe^2+ + Auarrow_forwardtrue or falsearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY