
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please help solve this question, and please do not post random other answer because I want to learn and study this, Thank you
Transcribed Image Text:Attempt
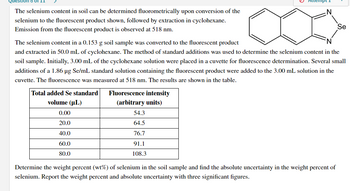
The selenium content in soil can be determined fluorometrically upon conversion of the
selenium to the fluorescent product shown, followed by extraction in cyclohexane.
Emission from the fluorescent product is observed at 518 nm.
Se
The selenium content in a 0.153 g soil sample was converted to the fluorescent product
and extracted in 50.0 mL of cyclohexane. The method of standard additions was used to determine the selenium content in the
soil sample. Initially, 3.00 mL of the cyclohexane solution were placed in a cuvette for fluorescence determination. Several small
additions of a 1.86 µg Se/mL standard solution containing the fluorescent product were added to the 3.00 mL solution in the
cuvette. The fluorescence was measured at 518 nm. The results are shown in the table.
Total added Se standard
Fluorescence intensity
volume (µL)
(arbitrary units)
0.00
54.3
20.0
64.5
40.0
76.7
60.0
91.1
80.0
108.3
Determine the weight percent (wt%) of selenium in the soil sample and find the absolute uncertainty in the weight percent of
selenium. Report the weight percent and absolute uncertainty with three significant figures.

Transcribed Image Text:Se wt% =
0.00174
Incorrect
%
+
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- answer part C/last question only. box the answer and MAKE SURE IT IS CORRECTarrow_forwardPHOTOSYNTHETIC PIGMENTS 1.6 0.7 Qy D 1.4 CH. 0.6 1.2 Soret 0.5 A N- 0.4 0.8 H COOCH3 0.3 0.6 phytyl bacteriochlorophyll a 0.2- 0.4 0.1 0.2 - 300 400 500 600 700 800 700 750 800 850 900 2 (nm) 2 (nm) Figure 4.7 Absorption (left) and fluorescence (right) spectra of bacteriochlorophyll a in diethyl ether. 5. Look at this figure from Blankenship's book, Molecular Mechanisms of Photosynthesis. You can see the structure for bacteriochlorophyll a, along with its absorption spectrum. a. What is the molecular shape around the labeled carbon atom? b. What is the hybridization around that same carbon? c. Based on this structure, would you expect this molecule to absorb light in the visible spectrum? Why or why not? Absorbance Fluorescence (Arbitrary units)arrow_forwardG neural crest tissue - Google Sear x A CHM 112 112-1 and 112-E1 A Presentation Session Student + 8 https://app.peardeck.com/student/twkkhzvar For quick access, place your favorites here on the favorites bar. Manage favorites now Peardeck Exercises Use #3. Nitol6) + 3 Fa(6)Z NF(1) + 3 HF(G) [1: 5,0 x10°n 0.10M Calculat K 2.0M 3.5 x 10°M Pear Deck Interactive Slide Students, draw anywhere on this slide! Do not remove this bar Slide 1/2arrow_forward
- Please specify what the correct answer is.arrow_forwardEnergy Respond to this prompt using 10 sentences or less. 1.) Give an example of kinetic energy in your daily life. Explain why your example is kinetic energy. 2.) Give an example of potential energy in your daily life. Explain why your example is potential energy. 3.) Provide an example where you observe either kinetic energy converting to potential energy or potential energy converting to kinetic energy.arrow_forwardThe above solution was wrong when entered into homework sitearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY