
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Determine the molecular structure of the following compound (C5H7NO2) using the data below
Please show how you determined the structure
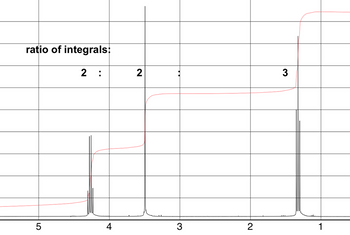
Transcribed Image Text:ratio of integrals:
LO
5
2:
4
2
3
2
3
1
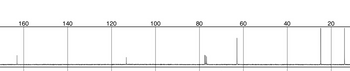
Transcribed Image Text:160
140
120
100
80
60
40
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Questions 6a, b, c, d is finked to one another and focus on the molecule shown below. O: H C2 H. I-Z:arrow_forwardThe functional group in the following molecule arearrow_forwardPlease send me the question in 20 minutes it's very urgent plz 8. Draw the Lewis structures for two resonance forms of the following moleculesions. Show any formal charges and determine which structure is major, which is minor, or if they are equivalenz [HICCO] (acylium ion)arrow_forward
- That’s the question and it’s chemistry not physicsarrow_forwardFrom the table of data below, calculate all of the interatomic equilibrium potential energies responsible for the molecule, methanol (CH3OH). Molecule/Radical/Atom CH3OH CH₂OH CH3O CH3 OH Н Enthalpy of Formation / Kcal mol¹ @ 298.15 K -48.1 -2.0 4.1 35.1 9.0 52.1arrow_forwardBased on the bond energies for the reaction below, what is the enthalpy of the reaction? HC=CH (g) + 5/2 O2 (g) → 2 CO2 (g) + H2O (g) Single Bond H C N H. 432 C 411 346 N 386 305 167 459 358 201 142 C=C 602 C=O 799 C=C 835 C=0 1072 Multiple Bonds C=N 615 O=0 494 C=N 887 N=N 942 **All values in kJ/mol**arrow_forward
- Electron-Dot Formulas and Shapearrow_forwardFill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. name of compound molecular formula | C1,0, CL,0, PCL, PCI, NH3arrow_forwardDetermine the structures of the molecules described in the following problems. Also note, in the combustion of N containing compounds, N2(g) is made. Using your understanding of bond enthalpies, predict the enthalpy change associated with the conversion of the amino acid lysine to cadaverine (and any other by-products of the reaction). You will need to have a balanced chemical reaction to do this. Assume that the reaction occurs in the gas phase.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY