
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:#
3
7
*
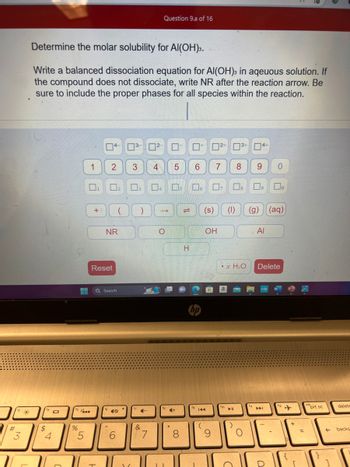
Determine the molar solubility for Al(OH)3.
Write a balanced dissociation equation for Al(OH)3 in aqeuous solution. If
the compound does not dissociate, write NR after the reaction arrow. Be
sure to include the proper phases for all species within the reaction.
$
4
101
is
%
1
5
▬
+:
04-03- 0²-
2
Reset
‒‒ Q Search
NR
₂
6
(
3
3
)
4-
&
7
Question 9.a of 16
4
O
II
5 6
+
8
s 16 7₂
H
(
72+ 13+
144
7
(s) (1)
OH
9
► 11
xH₂O
)
8 9 0
4+
7₂
8
O
Do
(g) (aq)
Al
Delete
prt sc
تاتان
delete
backs
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following aqueous solutions are good buffer systems? O 0.36 M hydrofluoric acid + 0.23 M potassium fluoride O 0.27 M hydrochloric acid + 0.22 M sodium chloride O 0.15 M potassium hydroxide + 0.24 M potassium chloride O 0.15 M hydrocyanic acid + 0.15 M sodium cyanide O 0.27 M ammonium bromide + 0.33 M ammoniaarrow_forwardFill out the tables and find pHarrow_forward3a) A student determines the concentration of a sodium hydroxide solution by titration with standardized KHP. S/he obtains the values: 0.190 M, 0.202 M, and 0.205 M. Should the value 0.190 M be rejected? Apply the Q Test. For three values Q must be greater than 0.94 to reject the number. Q = suspect nearest | largest - - smallest b) The student de to repeat the experiment two more The five values now include: 0.190 M, 0.202 M, 0.205 M, 0.201M and 0.203M. Use the Q Test to see if the first value may be rejected. For five values Q must be greater than 0.64 to reject the number. c) Solve for the average Molarity of the measurements from part b with and without the rejected number. Is there value in repeating an experiment several times?arrow_forward
- Determine the compound that is expected to be more soluble in acidic solution than in neutral or basic solution. О KBr O BaCl2 O Ni(OH)2 O Cu(NO3)2arrow_forwardWhat is the ratio of the moles of sodium hydroxide to the moles of crystal violet (nNaOH/nCV+) at the start of the reaction? Make sure to account for the fact that different volumes of the stock solutions are mixed. 5.0 mL 2.0 × 10–5 M crystal violet solution 1.0 mL of 0.10 M NaOH solution Trail. [Na+]j (M) [OH–]j (M) 1 1.67 × 10–2 1.67 × 10–2arrow_forwardDetermine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. NEXT > The buffer was prepared by dissolving 21.5 g HC,H,O₂ and 37.7 g of NaC,H,O₂ in 200.0 mL of solution. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) 0.178 0.880-x [0] [0.888] [1.31 + x) 0 0 0.888 1.31 + x HC₂H5O₂(aq) + [21.5] [x] Ka = 4.2 x 10-² 21.5 0.178 + x +x [37.7] [2x] 1 [0.178+x][0.888 + x] 37.7 -x 0.888 + x Determine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the pH. Complete Parts 1-3 before submitting your answer. 6.23 The Ka for HC₂H5O₂ is 6.3 x 10-5. Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka…arrow_forward
- 1. How do you prepare a 100mL of 0.1 M phosphate buffer?To make 100 mL of 0.1 M phosphate buffer: Calculate the amount of sodium phosphate needed. moles of sodium phosphate = (0.1 mol/L) x (0.1 L) = 0.01 moles mass of sodium phosphate = moles x molar mass = 0.01 moles x 142 g/mol = 1.42 g Dissolve 1.42 g of sodium phosphate in distilled water in a 100 mL volumetric flask. Adjust the pH of the solution to the desired value (usually around 7.4) using a strong acid or strong base. Bring the solution to the final volume (100 mL) with distilled water. 2. From the anterior buffer, how do you make 100mL of 0.05 M? To make 100 mL of 0.05 M phosphate buffer from the 0.1 M stock solution: Calculate the amount of the 0.1 M phosphate buffer needed. moles of phosphate buffer = (0.05 mol/L) x (0.1 L) = 0.005 moles Calculate the volume of the 0.1 M phosphate buffer needed. moles = concentration x volume (in liters) volume = moles / concentration = 0.005 moles / 0.1 mol/L = 0.05 L or 50 mL Measure…arrow_forwardA solution is prepared that is initially 0.27M in acetic acid (HCH3CO2) and 0.053M in potassium acetate (KCH3CO2). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [H₂O]. You can leave out the M symbol for molarity. [HCH2CO₂] cu_co, [H₂O*] initial change finalarrow_forwardThe silver-ion concentration in a saturated solution of silver(1) sulfate is 2.9 x 10-2 M. What is Ksp for silver(1) sulfate? 2.1 x 104 8.3 x 10-4 1.2 x 10-5 9.8 x 10-5 6.9 x 10-7arrow_forward
- The solubility product for silver (I) bromide is 7.7 × 10–13. Calculate the molar solubility of silver (I) bromide.arrow_forward5) Calculate the solubility of each of the following salts in moles per liter given their Ksp values. Ignore any acid-base properties.a. Ag3PO4, Ksp=1.8x10−18b. CaCO3, Ksp=8.7x10−9C) Calculate the solubility of the silver phosphate and the calcium carbonate in question 5 in grams/L.arrow_forwardI don’t know how to do this questionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY