
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Determine the frequency factor A for this reaction
Enter your answer in scientific notation to three significant figures
Temglates Symbols undo redo Teset keyboard shortcuts Help
A =
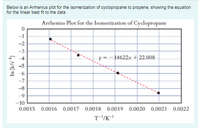
Transcribed Image Text:Below is an Arrhenius plot for the isomerization of cyclopropane to propene, showing the equation
for the linear best fit to the data.
Arrhenius Plot for the Isomerization of Cyclopropane
-1
=-14622x + 22.008
-5
-7
-8
-9
-10
0.0015 0.0016 0.0017 0.0018 0.0019 0.0020 0.0021 0.0022
T-/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain signal molecule S in muscle tissue is degraded by two different biochemical pathways: when only Path 1 is active, the half-life of S is 4.8 s. When only Path 2 is active, the half-life of S is 80.6 s. Calculate the half-life of S when both pathways are active. Rounded your answer to 2 significant digits. Пs S x10 X 5arrow_forwardCalculate the activation energy (in kJ) for a reaction whose rate constant becomes three times the initial value when the temperature is raised from 300 K to 310 K. Express your answer in decimal notation rounded to three significant figures. Type your answer..arrow_forwardEnter your answer in the provided box. Enter your answer in scientific notation. A first-order decomposition reaction has a rate constant of 0.00143 yr1. What is the half-life of the reaction? x 10 yrarrow_forward
- The data below were collected for the following reaction: CH3CI(g) + 3 Cl2(g) CCI4(g) + 3 HCI(g) Trial [CH3CI] / mol L-1 [Cl2] / mol L-1 Initial Rate / mol L-1 s-1 1 0.050 0.050 0.0142 2 0.100 0.050 0.0290 3 0.200 0.200 0.115 What is the rate constant?arrow_forwardSuppose a quantity is described by the function y(t) = 30,000e-0.05t, where t is measured in years. Find the half-life of the quantity.arrow_forwardA chemistry graduate student is studying the rate of this reaction: NH,OH (aq) → NH, (aq) +H,0 (aq) 4 He fills a reaction vessel with NH,OH and measures its concentration as the reaction proceeds: time [NH,OH] (milliseconds) 0.0500M 10. 0.0178M 20. 0.0108M 30. 0.00778 M 40. 0.00607Marrow_forward
- 1arrow_forwardRadioactive gold 198 is used in the diagnosis of liver problems. The half-life of the isotope is 2.70 days. If you begin with a 5.6 mg sample of the isotope, how much of the sample remains after 3.00 days?arrow_forward5. (Show your work) Some damage to the ozone layer of the upper atmosphere might involve the reaction NO + 03 NO2 + O2 The reaction is first order in each reactant and the rate constant is equal to 1.3 x 106 L/(mol s) at 298 K. What is the overall order of the reaction?arrow_forward
- The following stands for the frequency factor in the Arrhenius equation. Ea R A karrow_forwardThe rate law for a chemical reaction is given by: Rate = K[10³-]¹[1]²[H+]². Which statement is true? O O This reaction is first order with respect to 10³- and fifth order overall This reaction is first order with respect to 10³- and third order overall This reaction is third order with respect to H* This reaction is second order with respect to 1 and 3rd order overallarrow_forwardRadioactive gold-198 is used in the diagnosis of liver problems. The half-life of this isotope is 2.7 days. If you begin with a sample of 8.0 mg of the isotope, how much of this sample remains after 2.8 days? mgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY