
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Determine the following:a. Entropyb.RelativeEntropy.c. Redundancyc. Rate of Informationd. Efficiency
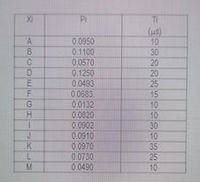
Transcribed Image Text:PI
(10)
10
30
20
20
25
A.
8.
0.0950
0.1100
0.0570
0.1250
0.0493
0 0683
0.0132
0:0820
0.0902
0.0910
15
10
10
30
10
K
0.0970
0.0730
0.0490
35
25
10
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 16. Calculate the number of solar thermal collectors of 2m2 area each, required to design a solar water heater that produce a 1000 kg/day of hot water with temperature of50oC from a cold water of temperature of 10oC, suppose the specific heat of water is 1.2 Wh/kg. K and the useful heat is 2400 Wh/m2/day: * Number of collectors 20. Number of collectors = 10. Number of collectors = 5. %3D None of the above.arrow_forwardCalculate for Period Cash Flow, PV Year 1, r=8%, Project Net Present Value (NPV) based on the provided.arrow_forwardGoal Solve for the efficiency of a heat engine using a five-step process the includes:1. Making a state table.2. Making a process table.3. Calculating the totals for Work, Heat, and Internal-Energy-Change.4. Identifying the heat input (hot reservoir) and output (cold reservoir).5. Calculating the efficiency of the engine.Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use 7.0 moles of nitrogen gas (diatomic). During the process a->b, the pressure rises by a factor of 2.0. solution- (1) Fill in the State Table (all pressures in Pascals, all volumes in cubic meters, all temperatures in K). Pressure Volume Temperature a b c (2) Fill in the Process Table (all entries in Joules). Work Heat dU a->b b->c c->a (3) Find the Totals: Work = J Heat = J dU = J (4) Find the heat input (from "hot reservoir") and the heat output (to…arrow_forward
- come with equations to solve for each variable while dealinng with constant pressure or it being reversible... w q delta U delta H isobaric isovolumetric (isochoric) isothermal (against constant pressure) isothermal (reversible) adiabatic (against constant pressure) adiabatic (reversible) complete cyclearrow_forwardSolve quick & Correctly, don't copy.arrow_forwardNOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A steam power plant receives heat from a furnace at a rate of 280 GJ/h. Heat losses to the surrounding air from the steam as it passes through the pipes and other components are estimated to be about 8 GJ/h. The waste heat is transferred to the cooling water at a rate of 170 GJ/h. Problem 06.017.a - Net power output of steam power plant Determine the net power output. (You must provide an answer before moving to the next part.) The net power output is MW.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY