
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
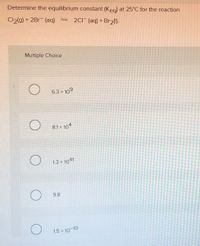
Transcribed Image Text:Determine the equilibrium constant (Keg) at 25°C for the reaction
Cl2(g) + 2Br (aq) S 2C1 (aq) + Br2().
Multiple Choice
6.3 × 109
8.1 × 104
1.3 x 1041
9.8
1.5 x 10-10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- Using enthalpies of formation, calculate AHrxn for the reaction: C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(()arrow_forwardHow many electrons are transferred in the following process, given the unbalanced reaction? PbO2 (s) + H* (aq) + Fe (s) → Fe3+(aq) + Pb2+ (aq) + H20 (1) Group of answer choices A-1 В-2 C-6 D-4 Е-3arrow_forward(1) Identify each of the following half-reactions as either an oxidation half-reaction or a reduction half-reaction. half-reaction Cl₂(g) + 2e →→→→2Cl¯(aq) Mg(s) Mg2+ (aq) + 2e¯ identification oxidation reduction (2) Write a balanced equation for the overall redox reaction. Use smallest possible integer coefficients. + +arrow_forward
- The equilibrium constant, K., for the following reaction is 9.52×10-2 at 350 K. CH4 (g) + CC14 (g) =2 CH2Cl2 (g) Calculate the equilibrium concentrations of reactants and product when 0.277 moles of CH, and 0.277 moles of CCl, are introduced into a 1.00 L vessel at 350 K. [ CH4] M [CC4] M [ CH2C1, ] = Marrow_forwardCalculate the ΔG^O (in kJ/mol) for the following reaction at 25.0℃.3Co^+2(aq)+2Al(s)→3Co(s)+2Al^+3(aq)arrow_forward2) Copper reacts with iodine in a similar reaction from what was done in lab to produce copper(I) iodide, CuI. The balanced equation for this reaction is 2Cu(s) + I2(s) ⟶2CuI(s) For this reaction, students obtain the following data: Mass I2: 0.5713 g Moles I2: 0.002251 mol Mass Cu: 0.3517 g Moles Cu: 0.005535 mol Which is the limiting reactant? Group of answer choices Cu I2 No answer text provided. No answer text provided.arrow_forward
- Nonearrow_forwardConsider the following reactions: CaSO4(s) Ca²+ + SO4²- CaF₂(s) Ca²+ + 2F- Kso=10-4.59 Kso-10-10.3 a) What is the solubility of CaSO4(s) in water at 25 °C? b) What is the solubility of CaF2(s) in water at 25 °C? c) Will either of these two solids will precipitate if [F]=104 M, [SO4²¯]=10-³ M and [Ca²+]=10-² M?arrow_forwardConsider the following reaction: 4PC13(g) P4(g) + 6C12(g) If the initial concentration of PC13(g) is 1.5 mol/L, and "x" is the equilibrium concentration of Pa(g), what is the correct equilibrium relation а. Кс-бх7 O b. Kc=6x7/(1.0-4x)4 O c. Kc=(x)(6x)6/(1.0-4x)4 O d. Kc=(x)(6x)°/(1.5-4x)4 O e. Kc=x'/(1.0-x)4arrow_forward
- Homework 3 - Chapter 5 - Thermochemistry Consider the reactions below: A2+2 C₂2 AC₂ A2+3 B22 AB3 2 B2+ C2 2 B₂C AH-150 kJ/mol AH-270 kJ/mol AH-280 kJ/mol Determine the enthalpy change for the following reaction using the information above. 4 AB3 +7C26 B₂C +4 AC2 AH: -480 Attempt: 4 (saved 2 minutes ago) 4444 7/11 tasks atten kJ/mol SAVE RESPONSE together to form thearrow_forwardUsing the information provided, determine the enthalpy (in kJ/mol) for the reaction 2 K(s) + 2 H₂O(l) → 2 KOH (aq) + H₂(g) K (s) = 0 kJ/mol H2O(l) = -285.8 kJ/mol KOH(aq) = -482.4 kJ/mol H2(g)= 0 kJ/molarrow_forwardConversion of cis-2-butene to trans-2-butene is represented by the equation kc=trans/Cisco. Calculate the equilibrium constant when 3mol of the cis-isomer in 8L is heated to 500 Celsius and at equilibrium the trans-ionomer is 2.1mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The