
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:1
4
7
+/-
Question 26 of 26
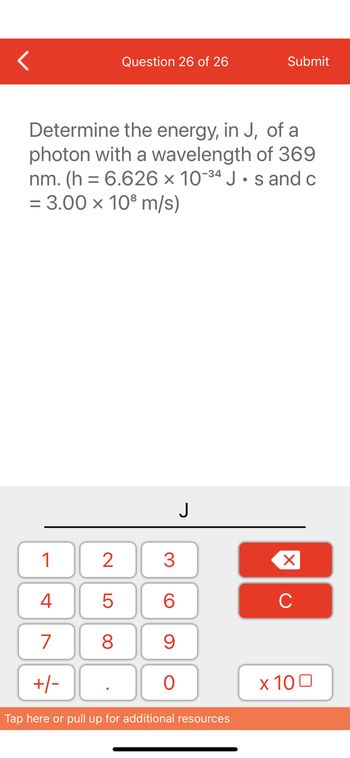
Determine the energy, in J, of a
photon with a wavelength of 369
nm. (h= 6.626 x 10-34 Js and c
= 3.00 x 10³ m/s)
2
5
8
3
60
9
O
J
Submit
Tap here or pull up for additional resources
XU
x 100
Expert Solution
arrow_forward
Step 1
Given :-
Wavelength of photon = 369 nm
h = 6.626 × 10-34 J•s
c = 3.00 × 108 m•s-1
To be calculated :-
Energy of photon
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the frequency of a photon if the energy is 7.59 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • s)arrow_forwardWhat is the wavelength (in nm) of a photon if the energy is 7.21 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • s)arrow_forwardWhat is the wavelength (in nm) of a photon if the energy is 7.55 × 10⁻¹⁹ J? (h = 6.626 × 10⁻³⁴ J • s)arrow_forward
- Calculate the frequency in hertz of electromagnetic radiation that has a wavelength of 576.0 nm. (c = 3.00 X 10⁸ m/s)arrow_forwardCalculate the energy of a 532 nm wavelength photon. h = 6.626 × 10-34 Js c = 3.00 × 108 m/sarrow_forwardWhat is the energy of a photon with a frequency of 5.40 × 10¹⁴ s⁻¹? (h = 6.626 × 10⁻³⁴ J • s) Calculate the frequency in hertz of electromagnetic radiation that has a wavelength of 584.0 nm. (c = 3.00 X 10⁸ m/s) Among the following H atom transitions, which would emit a photon of light with the longest wavelength?arrow_forward
- Determine the energy, in J, of a photon with a wavelength of 347 nm. (h= 6.626 x 10-34 Js and c = 3.00 x 10³ m/s)arrow_forwardCalculate the frequency in hertz of electromagnetic radiation that has a wavelength of 685.0 nm. (c = 3.00 X 10' m/s)arrow_forwardA photon has a frequency of 8.63 × 10⁸ Hz. What is the energy of this photon? (h = 6.626 × 10⁻³⁴ J • s)arrow_forward
- Calculate the frequency in hertz of electromagnetic radiation that has a wavelength of 585.0 nm. (c = 3.00 X 10º m/s)arrow_forwardCalculate the frequency in hertz of electromagnetic radiation that has a wavelength of 615.0 nm. (c = 3.00 X 10⁸ m/s)arrow_forwardAn electron in a hydrogen atom jumps from n=7 to n = 5 and emits a photon. What is the wavelength of the emitted light in the unit of nanometer? R=1.097×107 m-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY