
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
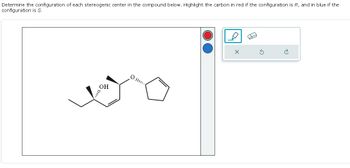
Transcribed Image Text:Determine the configuration of each stereogenic center in the compound below. Highlight the carbon in red if the configuration is R, and in blue if the
configuration is S.
OH
O.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound 1 Br Bri НІ T ... H The correct IUPAC names are: Compound 2 H Br Br I.. H Br The compounds are diastereomers not isomeric enantiomers identical constitutional isomers Compound 1: (2R, 3R)-1,2,3-tribromobutane, Compound 2: (2S, 3R)-1,2,3-tribromobutane Compound 1: (2R, 3S)-1,2,3-tribromobutane, O Compound 1: (2R,3R)-1,2,3-tribromobutane, Compound 2: (2S,3S)-1,2,3-tribromobutane Compound 2: (2R, 3R)-1,2,3-tribromobutane O Compound 1: (2S,3S)-1,2,3-tribromobutane, Compound 2: (2R,3S)-1,2,3-tribromobutanearrow_forwardAssign the absolute configuration of the asymmetric carbon in this structure as R or S. Br There is no asymmetric carbon in this structure OR Sarrow_forwardName the following compound. Inlcude cis/trans, R and S configuration if needed.arrow_forward
- 5. When cyclohexane is in the chair conformation, explain the difference between axial position and equatorial position. Hand-drawn the chair conformation and place the hydrogens (using different color) to axial and equatorial. 1 6. Draw a chair conformation of 1,4-dimethylcyclohexane in which on methyl group is equatorial and the other is axial. Draw a ring flip. From the drawing, determine whether it is a trans or cis. (Be sure to number all carbons for clarification).arrow_forwardNonearrow_forward8) For each of the pairs of molecules drawn below, place letters in the corner boxes corresponding to all accurate descriptions of the relationship between the two molecules. a) b) c) HO F A enantiomers F all letters that apply H H OH Вад CI all letters that apply all letters that apply B diastereomers C different conformations of the same molecule D identical conformations of the same molecule E constitutional isomers F stereoisomers G different molecules H both are chiral I neither is chiralarrow_forward
- just problem 5.26 pleasearrow_forwardWhich of the following compounds contain stereocenters? Also, what are stereocenters?arrow_forwardThere are nine constitutional isomers of molecular formula C7H16. 1) What is the unsaturation number of each of these compounds? 2) Draw five constitutional isomers, where two of these are chiral compounds. 3) For the three achiral constitutional isomers, provide the correct IUPAC names. 4) For the two chiral constitutional isomers, draw in Fischer projection formula the enantiomers of each. 5) Name, according to IUPAC standards, each of the enantiomers you drew in #4 above.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY