
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Determine K of the weak base B whose conjugate acid HB* has K₂ 8.3 × 10-4.
x 10
Determine the K₂ of the weak acid HA whose conjugate base has K₁ 2.7 × 10-8.
x 10
Expert Solution
arrow_forward
Step 1
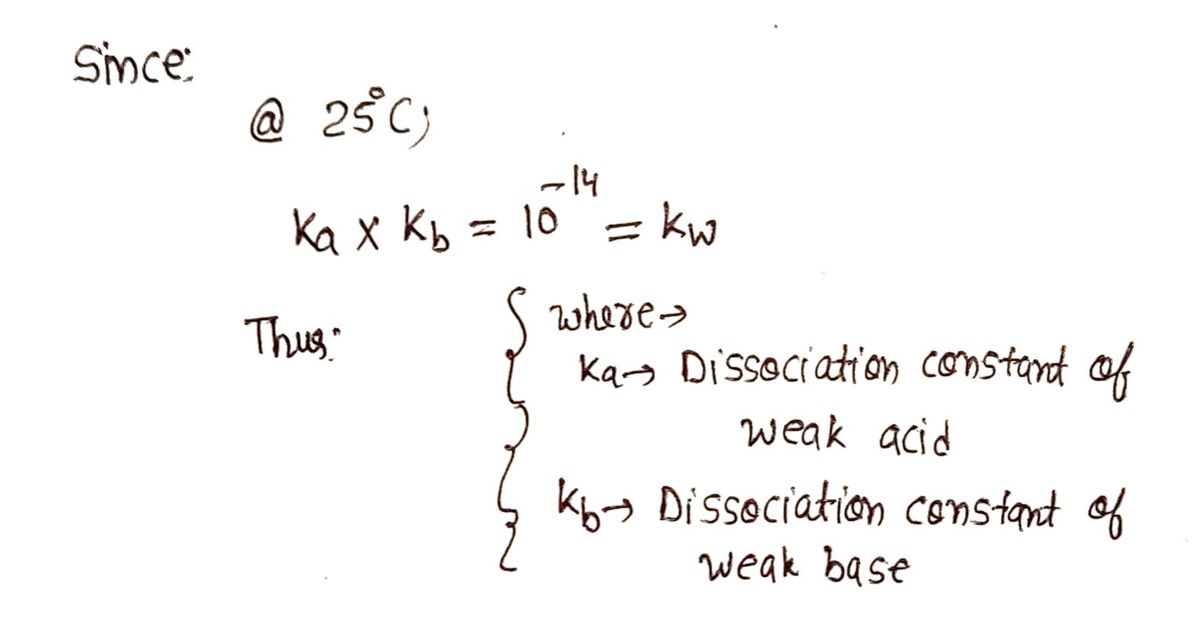
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH of a 0.14 M ammonium chloride, NH4Cl, solution. The Kb of ammonia, NH3, is 1.8 × 10−5 M.arrow_forwardWhich of the following two acids has the stronger conjugate base? Formic acid, Ka = 1.80 x 10–4 Hypochlorous acid, Ka = 3.0 x 10–8 Formic acid, because it has a smaller Ka Formic acid, because it has a larger Ka The strength of the conjugate base cannot be obtained from the information given. Hypochlorous acid, because it has a smaller Ka Hypochlorous acid, because it has a larger Kaarrow_forwardA typical aspirin tablet contains 325 mg acetylsalicylic acid. Calculate the pH of a solution that is prepared by dissolving 2 aspirin tablets in enough water to make a 269 mL solution. Assume the aspirin tablets are pure acetylsalicylic acid, K₂ = 3.3 x 10-4. Acetylsalicylic acid has a molar mass of 180 g/mol. pH = Submit Questionarrow_forward
- The pH of a 1.5 M solution of butanoic acid (HC H,O, is measured to be 2.32. Calculate the acid dissociation constant K of butanoic acid. Be sure your answer has the correct number of significant digits. K. = 0arrow_forwardA weak base has K, = 2.2 × 10-11. What is the value of K, for the conjugate acid? K.arrow_forwardG The pH of a 0.18M solution of hypobromous acid (HBrO) is measured to be 4.69. Calculate the acid dissociation constant K of hypobromous acid. Round your answer to 2 significant digits. K₁ = ⇓) x10arrow_forward
- The acid dissociation constant Ka of alloxanic acid HC4H3N2O5 is ×2.2410−7 . Calculate the pH of a 3.2M solution of alloxanic acid. Round your answer to 1 decimal place.arrow_forwardA solution is made by dissolving 25.1 g of Ba(NO₂)₂ in 500.0 mL of water. Using Kb(NO₂⁻) = 2.2 × 10⁻¹¹, determine the pH of the solution.arrow_forward2,4,7,8arrow_forward
- Calculate the pH of a 0.41 M CH₂COOLi solution. (K for acetic acid=1.75 × 10¯5.) Be sure your answer has the correct number of significant digits. x10 X Śarrow_forwardK, for formic acid, HCOOH, is 1.80x10-4. Ka for acetylsalicylic acid (aspirin), HC,H,04, is 3.00x10-4. Ка Ką for nitrous acid, HNO2, is 4.50x10-4. What is the formula for the weakest conjugate base?arrow_forward9. Which of the following weak acids would have the STRONGEST conjugate base? hypochlorous acid (HClO) benzoic acid (C6H5COOH) hydrocyanic acid (HCN) hydrofluoric acid (HF) nitrous acid (HNO2)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY