
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Describe the mechanism shown for a double zinc aminopeptidase. The image shows the catalysis pathway of double zinc aminopeptidase
- Describe the chemistry of each step (what is happening in each step/pathway)
- How the enzyme appears or might facilitate the chemistry
- How the enzyme increases the reaction rate..
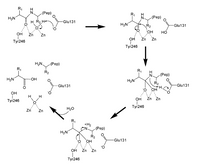
Transcribed Image Text:R1
H
(Реp)
R1
(Pep)
H2N
R2
-Glu131
H.
H2N'
R2
Glu131
"OH
но
он
Zn
он Zn
Zn
Tyr246
Tyr246
H2N,
-(Реp)
R1
R1
R2
(Рер)
H2N
-OH
H2N
R2
-Glu131
Glu131
он
Он
он
Zn Zn
Tyr246
Tyr246
H20
Zn
Zn
R1
+H2
-(Pep)
H2N
он
-Glu131
OH Zn Zn
Тyr246
IZ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Answering Subpart 1 - Establishing the concept and chemistry of the enzyme hydrolysis
VIEW Step 2: The attack of OH- from water on the peptide carbonyl carbon
VIEW Step 3: Formation of the tetrahedral complex
VIEW Step 4: The splitting of the amide bond being set up
VIEW Step 5: The final split of the amide bond
VIEW Step 6: Answers to 2nd and 3rd subpart of the Question
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (d) On the other hand, in quantum physics/mechanics/chemistry, energy is assumed to be quantized and distributed. Therefore, virtually any reaction can take place in a cell even though some reactions remain exceedingly slow (or not possible on the time-scale of life processes) without enzyme catalysis. Explain why.arrow_forwardPractice 14: The following is a schematic of an active site in an enzyme. Considering the shape and electronic characteristics of the active site, suggest and draw a possible substrate that would be electronically complimentary. Show and describe the forces that hold the substrate to the active site. HN "NH₂ NHarrow_forwardWhat chemical reaction is being catalyzed in this experiment? Label the substrate(s), enzyme and product(s). Based on the information in the data table and your graph, is there an optimum temperature for catalase that makes it the most productive in terms of reaction rate? If so, what is the temperature? 3. At what temperature, does the rate of the reaction decrease? Explain why.arrow_forward
- Explain enzyme kinetics including zero-order and first-order kinetics.arrow_forwardThe enzyme examase catalyses the following reaction, A - B where A is the reactant and B is the product A595 measurements were used to determine the catalytic activity of examase by measuring the rate at which B is produced. From this, the activity of the enzyme was determined to be 42 µmol/mL per minute. A standard albumin solution (concentration = 10 mg/mL) was used to prepare a standard curve of Abs595 vs amount of protein in mg. The protein assay was also performed using 100 uL of a 1 in 10 dilution of the original examase sample. For the absorbance measurements, it was determined that the sample used in the A595 measurement contains 1.2 mg of examase. Which of the following statements correctly gives the specific activity of examase? 1.0.35 U/mg O 2.0.035 U/mg 3. 5040 U/mg O 4.35 U/mg O 5.3.5 U/mgarrow_forward5 of 5 7. When a catalyst is added to a reaction the activation Energy is (increased or decreased), (more or less) energy is needed to react the new activated complex, and (more or less) collisions have sufficient energy to (increase or decrease) the reaction rate.arrow_forward
- Using this graph, what can be determined about the effect of enzyme concentration on the initial rate of the reaction and on the amount of product formed?arrow_forwardwhat is the approximation used to derive the reaction rate for an enzyme catalysed reaction that proceeds by a michaelis menten mechansimarrow_forwardThe acid-catalysed conversion of γ-hydroxybutyric acid (GHBA) into its lactone, γ-butyrolactone (GBL) is a reversible reaction. The GHBA to GBL forward reaction is first-order with respect to the GHBA concentration; the GBL to GHBA reverse reaction is first-order with respect to the GBL concentration. An experimental study of the kinetics of this reaction was undertaken in 0.2 mol L-1hydrochloric acid (HCl) at 298 K. The initial concentration of GHBA was 18.23 × 10-3mol L-1 . The concentration of GBL in solution was followed as a function of time (t), as indicated in Table B.3: Time/min 0 21 36 50 65 80 100 ∞ GBL Concentration / 10-3 L-1 0 2.41 3.73 4.96 6.10 7.08 8.11 13.28 Use the data in Table B.3 to determine the equilibrium constant and the first-order rate constants for both forward and reverse reactions.arrow_forward
- Do not answer in image format. Maintain accuracy and quality in your answer. Answer completely.arrow_forwardWhy do most living organisms have an enzyme to decompose hydrogen peroxide? Ideal Gas and Decomposition of Hydrogen Peroxide Quiz Because hydrogen peroxide is present in many foods. Because hydrogen peroxide is toxic and needs to be destroyed. Because hydrogen peroxide does not dissolve in water. Because hydrogen peroxide is produced in the liver.arrow_forwardWhat solution asap....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY