
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
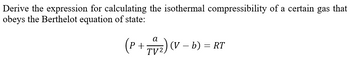
Transcribed Image Text:Derive the expression for calculating the isothermal compressibility of a certain gas that
obeys the Berthelot equation of state:
a
(P + 74₂2) (1
(V-
TV2
(V - b) = RT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- The fugacity of an ideal gas is found to obey f(T, P) =P exp(bT), where b is a constant with units of inverse temperature. find Cp.arrow_forward6 ref book: Introduction to Chemical Engineering Thermodynamics (Van ness)arrow_forward* Your answer is incorrect. A rigid tank whose volume is 0.5 m², initially containing ammonia at 20°C, 1.5 bar, is connected by a valve to a large supply line carrying ammonia at 12 bar, 130°C. The valve is opened only as long as required to fill the tank with additional ammonia, bringing the total mass of ammonia in the tank to 136.7 kg. Finally, the tank holds a two-phase liquid-vapor mixture at 20°C. Determine the heat transfer to the ammonia in the tank from the surroundings, in kJ, ignoring kinetic and potential energy effects. Qev = i -1677404.2311 kJarrow_forward
- Determine pressure of CO2 at 400 °C with a density of 10 mol/l. Using (a) The ideal-gas equation. (b) The van der Waals equation. (c) The generalized correlation of the 2nd-order virial coefficient. CO₂: Tc = 304.2 K, Pc = 73.83 bar, o = 0.224 and molecular weight 44 g/mol.arrow_forward1. Calculate AU, AH, q, and w for the reversible isothermal expansion of 62.4 g of Ar from a volume of 2.50 L to 15.5 L at 150.0 K. a. Treat Ar as an ideal gas. b. Treat Ar as a gas with the equation of state given below where a represents the van der Waals attraction constant. (We are ignoring the constant b in this problem.) а (P+ -)V = RT V? m т c. Explain any difference between your answers to parts a and b.arrow_forwardDerive an expression for the isothermal compressibility of a gas that obeys the Dieterici EOS. The Dieterici EOS is given by: a RTE¯RTV P = V - barrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The