
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
(Anyone) Can you please help me with this Chemistry problems? (all of them)
![Structures and abbreviations of the 20 standard amino acids in
classified according to their side chain (R group) chemistry
form,
Hydrophobic amino acids
COO™
H-C-CH3
NH
Alanine (Ala, A)
Model 2:
witterionic
COO™
H-C-CH₂-CH
NH
CH₂
Leucine (Leu, L)
COO™
Toulon H-C
NH
-CH₂-
Tryptophan (Trp, W)
Table Polar amino acids
COO™
H-C-CH₂-OH
NH
Serine (Ser, S),
COO™
CH3
H-C
CH₂
O
H-C
NH
Asparagine (Asn, N)
COO™
-NH₂
-CH₂-SH
NH
10 xod and Cysteine (Cys, C)
NH
Aspartate (Asp, D)
COO™
H-C
Charged amino acids
COO™
Lo
H-C-CH₂-C-0-
NH
CH₂
aline (Val, V)
COO™
H-C
NH
H-C
CH3
-CH
St
Isoleucine (Ile, I)
-CH-CH₂-CH3
CH₂
COO™ CH3
COO™
CH₂
H-C-
H₂N
-CH₂
Proline (Pro, P)
COO
Houba
NH
Threonine (Thr, T)
CH₂
COO
-CH-OH
H-C-CH₂-CH₂-C-NH₂
ΝΗ;
Glutamine (Gln, Q)
COO
H-C-H
NH
Glycine (Gly, G)
O
Wa
H-C-CH₂-CH₂-C-O
COO™
NH₂
H–C—CH, —CH, —CH, NH—C=NH
ΝΗ;
NH
Glutamate (Glu, E)
COO™
H-C-CH₂-
NH
Phenylalanine (Phe, F)
COO™
H-C-CH₂-CH₂-S-CH₂
NH
Methionine (Met, M)
COO
H-C-CH₂
NH
- 247 -
Tyrosine (Tyr, Y)
COO™
H-C-CH₂
NH
H
Histidine (His, H)
N
-OH
COO
H—C—CH, CH, —CH, —CH, NH
ΝΗ,
Lysine (Lys, K)
D
Arginine (Arg, R)
[From Essential Biochemistry 3/e; Pratt, C.W. and Cornely, K.; Copyright © (2013) by John Wiley & Sons, Inc.
Reprinted with permission of John Wiley & Sons, Inc.]
Critical Thinking Questions:
5. Look at the structure of alanine at the top of Model 2. Identify the amino group and the
carboxyl group in alanine. Ensure that all team members agree.
CA46A](https://content.bartleby.com/qna-images/question/7207ec05-c570-45c5-9f93-6adc9f301e8e/aa1a0ca8-0688-457a-bbf4-374f3eba6edd/nc4nyvb_thumbnail.jpeg)
Transcribed Image Text:Structures and abbreviations of the 20 standard amino acids in
classified according to their side chain (R group) chemistry
form,
Hydrophobic amino acids
COO™
H-C-CH3
NH
Alanine (Ala, A)
Model 2:
witterionic
COO™
H-C-CH₂-CH
NH
CH₂
Leucine (Leu, L)
COO™
Toulon H-C
NH
-CH₂-
Tryptophan (Trp, W)
Table Polar amino acids
COO™
H-C-CH₂-OH
NH
Serine (Ser, S),
COO™
CH3
H-C
CH₂
O
H-C
NH
Asparagine (Asn, N)
COO™
-NH₂
-CH₂-SH
NH
10 xod and Cysteine (Cys, C)
NH
Aspartate (Asp, D)
COO™
H-C
Charged amino acids
COO™
Lo
H-C-CH₂-C-0-
NH
CH₂
aline (Val, V)
COO™
H-C
NH
H-C
CH3
-CH
St
Isoleucine (Ile, I)
-CH-CH₂-CH3
CH₂
COO™ CH3
COO™
CH₂
H-C-
H₂N
-CH₂
Proline (Pro, P)
COO
Houba
NH
Threonine (Thr, T)
CH₂
COO
-CH-OH
H-C-CH₂-CH₂-C-NH₂
ΝΗ;
Glutamine (Gln, Q)
COO
H-C-H
NH
Glycine (Gly, G)
O
Wa
H-C-CH₂-CH₂-C-O
COO™
NH₂
H–C—CH, —CH, —CH, NH—C=NH
ΝΗ;
NH
Glutamate (Glu, E)
COO™
H-C-CH₂-
NH
Phenylalanine (Phe, F)
COO™
H-C-CH₂-CH₂-S-CH₂
NH
Methionine (Met, M)
COO
H-C-CH₂
NH
- 247 -
Tyrosine (Tyr, Y)
COO™
H-C-CH₂
NH
H
Histidine (His, H)
N
-OH
COO
H—C—CH, CH, —CH, —CH, NH
ΝΗ,
Lysine (Lys, K)
D
Arginine (Arg, R)
[From Essential Biochemistry 3/e; Pratt, C.W. and Cornely, K.; Copyright © (2013) by John Wiley & Sons, Inc.
Reprinted with permission of John Wiley & Sons, Inc.]
Critical Thinking Questions:
5. Look at the structure of alanine at the top of Model 2. Identify the amino group and the
carboxyl group in alanine. Ensure that all team members agree.
CA46A
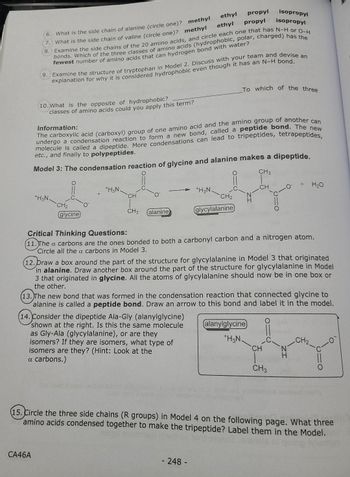
Transcribed Image Text:8
6. What is the side chain of alanine (circle one)? methyl
What is the side chain of valine (circle one)? methyl
ethyl
propyl
7.
bonds. Which of the three classes of amino acids (hydrophobic, polar, charged) has the
Examine the side chains of the 20 amino acids, and circle each one that has N-H or O-H
fewest number of amino acids that can hydrogen bond with water?
10. What is the opposite of hydrophobic?
classes of amino acids could you apply this term?
CA46A
9. Examine the structure of tryptophan in Model 2. Discuss with your team and devise an
explanation for why it is considered hydrophobic even though it has an N-H bond.
+H3N.
CH₂
(glycine)
Information:
The carboxylic acid (carboxyl) group of one amino acid and the amino group of another can
undergo a condensation reaction to form a new bond, called a peptide bond. The new
molecule is called a dipeptide. More condensations can lead to tripeptides, tetrapeptides,
etc., and finally to polypeptides.
+H3N.
ethyl
Model 3: The condensation reaction of glycine and alanine makes a dipeptide.
O
CH3
CH
CH3 alanine
+H3N.
propyl
CH₂
glycylalanine
248-
To which of the three
isopropyl
isopropyl
CH
Critical Thinking Questions:
(11.) The a carbons are the ones bonded to both a carbonyl carbon and a nitrogen atom.
Circle all the a carbons in Model 3.
(12. Draw a box around the part of the structure for glycylalanine in Model 3 that originated
in alanine. Draw another box around the part of the structure for glycylalanine in Model
3 that originated in glycine. All the atoms of glycylalanine should now be in one box or
the other.
alanylglycine
+H3N
(13. The new bond that was formed in the condensation reaction that connected glycine to
alanine is called a peptide bond. Draw an arrow to this bond and label it in the model.
(14. Consider the dipeptide Ala-Gly (alanylglycine)
shown at the right. Is this the same molecule
as Gly-Ala (glycylalanine), or are they
isomers? If they are isomers, what type of
isomers are they? (Hint: Look at the
a carbons.)
+ H₂O
CH
CH3
15. Circle the three side chains (R groups) in Model 4 on the following page. What three
amino acids condensed together to make the tripeptide? Label them in the Model.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 11 Which of the following E,Z-designations are correct? Mark all correct answers. CO.H H. CH-CH%3DCH2 CH-CH(CH32 H3C (E) Br CHANH2 CH NHCH, (Z} HOCH CH2OCH COCH3 (E} NC CH3 (CHNCH2 CH,CHa (E} Click Save and Submit to save and submit. Click Save All Answers to save all answers.arrow_forwardCan you work our KSP for me? thanks Liquid Amount - How many grams of each of the following substances will dissolve in 4.70×102 mL of cold water? Substance - The solubility is 0.123 g/100 mL at 20 oC. ( Ce(IO3)4 )arrow_forwardI need help with finding the distance component moved and distance solvent movedarrow_forward
- Need help with 2-5arrow_forwardQuestions 15 through 20 pertain to the following medication formula: Here is the formula a pain and inflammation reducing ointment. Methyl salicylate (sp gr = 1.167) 60 mL Lidocaine 4% (w/w) Tween:Span mix (1:4 mix) Polypeg Base 10 g QS to 0.5 kg You receive an order to make 3 solid ounces of this product. Answer the following questions pertaining to producing 3 solid ounces of the product. 15. Volume of methyl salicylate, in milliliters. 16. Weight of lidocaine in grams. 17. Weight of Tween in milligrams. 18. Weight of Span, in milligrams. 19. Weight of Polypeg base, in grams. 20. What is the final concentration of methyl salicylate, as a percent concentration (w/w)?arrow_forwardThe mystery fuel speedster! (Homework ) For fun, you build an explosive car with your little brother by attaching wheels to an empty windshield washer container. To advance it, you add 1.20 ml ( = 0.7915 g/ml) of a liquid but volatile fuel into the container. You close the cap and shake to evaporate the liquid. Finally, you place the car on its wheels, remove the cap and insert a burning wooden rod into the container. A flame suddenly comes out of the car, which is propelled with good speed in front of your little brother surprised, but all upset by the maneuver. The volatile fuel is composed of 37.5% carbon, 12.6% hydrogen and 49.9% oxygen. Its molar mass is approximately 32.0 g/mol. During the reaction, the fuel (gaseous) burns in the presence of gaseous oxygen, and the products of the reaction are carbon dioxide and water, gaseous too. The volume of the windshield washer container used is 4.00 litres. Useful Info: There is 21.0% gaseous oxygen in the air and initial conditions…arrow_forward
- 8 = filled CHM-202 Lab 1 Solids and Solutions Rev 1 G6-2022 Results: 1. Models of Crystalline Unit Cells Atomic Radius (radius of styrofoam ball) Volume of one atom (one ball, 4/3r¹) Measured unit cell length (1) [1.3] No. Atoms per cell [1.3] Ratio of 1/r [1.3] Geometric unit cell length (1) [1.5a] Volume of atoms in cell [1.5c] Total 3 Volume of Unit Cell [1.5c] Percent vol filled [1.5d] Percent free space. [1.5d] I atom Simple Cubic SC 6.15mm 118 6.9 172 Raw Data (Temperature vs. Time) Trial Time CHM-202 Lab 2 Freering Point Depression Rev2 06-2022 232.6 Temp Time d= 6.9 r = 3.24 Sum v=172²³² Body Centered Cubic BCC 8 lan a atom 2.3 7.96 344 531.45 B. Cyclohexane Solutions (Trial 4 if needed) Temp Time (include units!) (include units!) Face Centered Cubic FCC (CCP) 10.8cm 4 atom 7/12 3.13 Temp 5.26 688 1259-73 Time the pieces are not Temparrow_forwardi need help calculating slope and inverse slope pleasarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY