
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
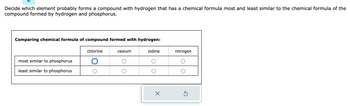
Transcribed Image Text:**Task:**
Decide which element probably forms a compound with hydrogen that has a chemical formula most and least similar to the chemical formula of the compound formed by hydrogen and phosphorus.
**Table: Comparing chemical formula of compound formed with hydrogen:**
| | Chlorine | Cesium | Iodine | Nitrogen |
|---------------------------|----------|--------|--------|----------|
| Most Similar to Phosphorus | ◯ | ◯ | ◯ | ◯ |
| Least Similar to Phosphorus| ◯ | ◯ | ◯ | ◯ |
**Explanation:**
The table displays a selection mechanism where different elements (chlorine, cesium, iodine, nitrogen) are evaluated for how similar their compounds with hydrogen are to that with phosphorus. These elements can be marked as either most similar or least similar using provided selection bubbles.
Expert Solution
arrow_forward
Step 1
Similarity of Hydrogen with phosphours :- It formed PH3 compounds
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the element flowchart for Si: HCI Si NaOH CH3CI Na₂CO3 SiO₂ HF C H+arrow_forwardlon Commonly Formed Name of element SYMBOL Number of Number of electrons in ion protons in ion fluorine Ga 12 potassium Sarrow_forwardDetermine the Average Atomic Mass of the mixtures of Nitrogen isotopes 95% 1“N, 3% 15N, and 2% 1®N 14 amu 13.3 amu 14.07 amu 15 amuarrow_forward
- Decide which element probably forms a compound with hydrogen that has a chemical formula most and least similar to the chemical formula of the compound formed by hydrogen and fluorine. Comparing chemical formula of compound formed with hydrogen: chlorine sulfur strontium selenium most similar to fluorine least similar to fluorinearrow_forwardWhen carbonic acid gas is allowed to react with lithium hydroxide it produces lithium carbonate. Lithium carbonate is used to treat depression and bipolar disorder. Write chemical formulas for carbonic acid, lithium hydroxide and lithium carbonate.arrow_forwardCompare atomic size (<, >, or =): Cu ___ Ag Te ___ Se Na ___ Na+ O ___ O2–arrow_forward
- Which choice(s) of the following have 18 electrons O Scandium3+ O Calcium O Argon Argon ion O Chlorine O Siliconarrow_forwardElement X reacts with element Y to give a product containing X2+ ions and Y− ions. Complete the sentences to identify whether element X or Y is likely to be a metal or a nonmetal What is the formula of the product?arrow_forwardMatch each element with the charge it will have when it becomes an ion. nitrogen V xenon v magnesium v bromine v rubidium v selenium aluminumarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY