
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
| If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. If two oxygen atoms don't satisfy the octet rule, enter "O,O". |
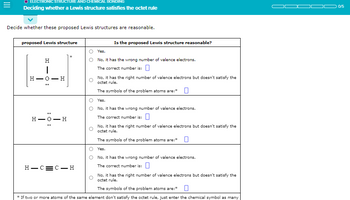
Transcribed Image Text:ELECTRONIC STRUCTURE
Deciding whether a Lewis structure satisfies the octet rule
Decide whether these proposed Lewis structures are reasonable.
proposed Lewis structure
H
HIO
|
H-
-0
- H
H-O-H
H-C=C-H
Is the proposed Lewis structure reasonable?
Yes.
No, it has the wrong number of valence electrons.
The correct number is: 0
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
No, it has the wrong number of valence electrons.
The correct number is: 0
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*
Yes.
No, it has the wrong number of valence electrons.
The correct number is: 0
No, it has the right number of valence electrons but doesn't satisfy the
octet rule.
The symbols of the problem atoms are:*0
* If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many
0/5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis structures of the neutral atom of Aluminum and the ion of Aluminum. Determine the number of electrons gained or lost in forming an ion.arrow_forwardAmmonium bicarbonate, NH4HCO3, contains both ionic and covalent bonds. Draw the Lewis structure of this compound. Explicitly draw all H atoms. • Draw cations and anions in separate sketchers. Do not use the square brackets tool in your answer. opy astearrow_forward* If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as necessary. if two oxygen atoms have the wrong number of electrons around them, enter the symbol O twice.arrow_forward
- To draw a Lewis Structure, start with the atomic symbol in the middle, then put dots around the symbol until all of the valence electrons are represented. Try to envision a box around the atom symbol, and the electrons on each side of the box. The valence electrons should be drawn around the element symbol one on each side before pairing the electrons up. The maximum number of electrons that could be on one side of a Lewis Structure is two, and the maximum number of electrons around an element symbol is eight. To figure out how many valence electrons each element has, use the Periodic Table. Remember, the number of valance electrons for Group A elements is equal to the Group A number the element is in on the Periodic Table. Use the data you collected in Table 1 to a draw Lewis structure model to show the formation of any one of the compounds you assembled, then explain the model you drew. Remember, you should be explaining and supporting your answers by referencing and citing the…arrow_forwardDraw the Lewis structure for OSiS before answering the following questions (You do NOT submit a picture just answer the questions). Silicon is the central atom and all atoms obey the octet rule. WARNING: Do NOT USE the internet or other sources to find the structures. Use ONLY the rules taught in class. ALL atoms obey the octet rule Answer the following questions for the Lewis structure for OSiS , given that silicon is the central atom and all atoms obey the octet rule. Give answers as numbers (1,2,3 ...etc.) NOT words (one, two three etc.) How many double bonds exist in this structure How many electrons surround the silicon atom How many lone pairs are around the silicon atom How many lone pairs are around the sulfur atom How many lone pairs are around the oxygen atom How many electrons surround the Sulfur atomarrow_forwardBonding where electrons are shared equally between two or more atoms is which of the following? ionic non polar covalent hydrogen polar covalentarrow_forward
- diagram of a molecule that shows atoms based on their a periodic table symbols. These symbols are surrounded by pairs of dots to show valence electrons that could be lone pairs or bonding pairs?arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: Н — Н — О No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0=0= O No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: Н — СС — Н No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0". :0: :0: :0 :arrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forward
- Main group metals lose electrons, resulting in the electron configuration of the previous noble gas. Nonmetals gain electrons, resulting in the electron configuration of the nearest noble gas. Transition metals do NOT follow the Octet rule True or false?arrow_forwardTo draw a Lewis Structure, start with the atomic symbol in the middle, then put dots around the symbol until all of the valence electrons are represented. Try to envision a box around the atom symbol, and the electrons on each side of the box. The valence electrons should be drawn around the element symbol one on each side before pairing the electrons up. The maximum number of electrons that could be on one side of a Lewis Structure is two, and the maximum number of electrons around an element symbol is eight. To figure out how many valence electrons each element has, use the Periodic Table. Remember, the number of valance electrons for Group A elements is equal to the Group A number the element is in on the Periodic Table. Use the data you collected in Table 1 to a draw Lewis structure model to show the formation of any one of the compounds you assembled, then explain the model you drew. Remember, you should be explaining and supporting your answers by referencing and citing the…arrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY