
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
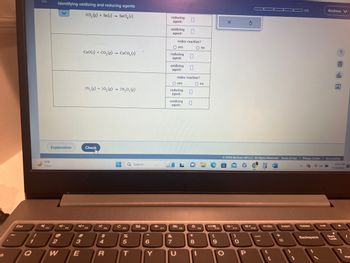
Redox : identifying oxidation and reducing agent
Transcribed Image Text:Esc
1
Q
Identifying oxidizing and reducing agents
2CL₂(g) + Sn(s) SnCl₂ (s)
Explanation
71°F
Clear
2
W
4+
3
E
CaO(s) + CO,(g) → CaCO, (s)
2N₂ (g) +50₂(g) → 2N₂O, (g)
-
Check
X
F4
$
4
1
R
▬▬▬ Q Search
-
F5
15
%
5
T
F6
6
Y
reducing
agent:
oxidizing
agent:
redox reaction?
O yes
reducing
agent:
oxidizing 0
agent:
&
7
O yes
reducing
agent:
oxidizing
agent:
U
redox reaction?
0
F8
0
LO
O no
O no
8
*
8
F9
1
C
F10
(
9
X
O
*-
F11
)
O
S
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
**+
P
F12
Prisc
[
Insert
=
0/5
1
G
Delete
Backspace
x 5
Andrew
14
?
olo
Ar
9:24 PM
7/19/2023
Num
Lock
7
I
Won
Transcribed Image Text:Esc
← → C
1
=
71°F
Clear
20
FI
Significant Figures Calculator and XM (no subject) - enterprise1701dd x
+
www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvgXwPgmUhvlTCeeBZbufuBYTI0Hz7m7D3ZSIqFYZDOBV6RpfMn8AQdHPIcZmyNDCsAtAPcBiyNq... 12
O CHEMICAL REACTIONS
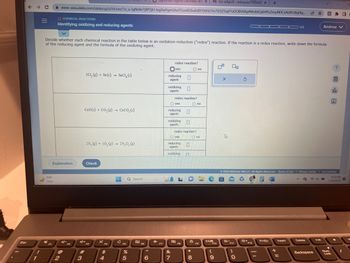
Identifying oxidizing and reducing agents
Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the formula
of the reducing agent and the formula of the oxidizing agent.
Explanation
@
2
2C1, (g) + Sn(s) SnCl, (s)
CaO(s) + CO,(g) → CaCO, (s)
4+
#
3
2N₂ (g) +50₂ (g) 2N₂O, (g)
Check
F4
$
4
HE
с
F5
Q Search
%
5
F6
6
✈
F7
redox reaction?
yes
reducing
agent:
oxidizing
agent:
1644
O yes
redox reaction?
reducing
agent:
oxidizing
agent:
O yes
&
7
reducing
redox reaction?
agent:
oxidizing n
Ono
F8
O no
Ono
*
8
F9
DB
F10
(
9
X
4
00
*-
F11
3
0
*+
F12
00 0/5
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
G 745
9:23 PM
7/19/2023
-
PrtSc
Insert
E
Delete
Backspace
Andrew V
►/1
?
Lock
F ESEN
ola
Ar
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which method is used to make fake gold coins? Additive Plating: A plating reaction where metal cations are reduced by an electron donor in solution. The reduced cations coat the surface of an object. OR Displacement Plating A plating reaction is where metal cations are reduced by metals on the surface of an object. The reduced metal cations become neutral atoms and coat the object, replacing the oxidized metal atoms that become metal cations.arrow_forwardProvide two written definitions of an oxidation-reduction reaction and for each definition tell which reactant is reduced and which is oxidized. Then tell which of the two definitions is the most comprehensive.arrow_forwardOH Excess Oxidantarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY