
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
FIND THE TRIANGLE FOR BOTH IRON AND WATER PLEASE!
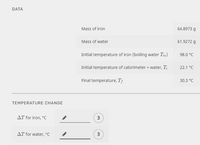
Transcribed Image Text:**Data:**
- **Mass of iron:** 64.8973 g
- **Mass of water:** 61.9272 g
- **Initial temperature of iron (boiling water \( T_m \)):** 98.0 °C
- **Initial temperature of calorimeter + water (\( T_i \)):** 22.1 °C
- **Final temperature (\( T_f \)):** 30.3 °C
---
**Temperature Change:**
- \( \Delta T \) for iron, °C
- \( \Delta T \) for water, °C
Note: The temperature change (\( \Delta T \)) fields each have an editable input option with a default value of 3.
Expert Solution
arrow_forward
Step 1
The solution is given below -
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Would ice (density = 0.934 g/mL) float in ethyl alcohol (density = 0.789 g/mL)? Explain.arrow_forwardDescribe the two characteristics of water that cause it to evaporate more slowly than other liquids. Use the paperclip button below to attach files. Student can enter max 3500 characters В I U Rain off and on e to searcharrow_forwardPlease help me solve the following question and make sure everything is correct !! thanksarrow_forward
- Item 10 10 of 33 Complete I Review | Constants I Periodic Table Part A The compound MgCl2 is named dimagnesium chloride. magnesium chlorine. magnesium (II) chloride. magnesium dichloride. magnesium chloride.arrow_forward1a. Determine the volume of 1.000 g of water (Densitywater = 0.9999 g/mL). 1b. Determine the volume of 1.000 g of ice (Density ce = 0.9168 g/cm³). (1 mL = 1 cm³) %3D %3D 1c. From the volumes above, what can we conclude about 1.000 g of water when it freezes?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY