
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hello, I'm having some trouble calculating the final mass of decsiccant, Mass of water in (g), and the mass % water in unknown hydrate. I did the first two but I didnt know if it was right to the correct significant figures. I need the last 3 boxes. Thank you.
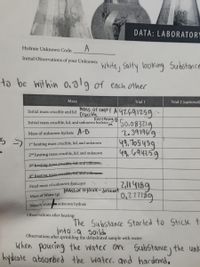
Transcribed Image Text:DATA: LABORATORY
Hydrate Unknown Code:
A
Initial Observations of your Unknown:
White, Salty looking Substance
to be within 0.019 of each other
Mass
Trial 1
Trial 2 (optional)
Initial mass crucible and lid o Cmpt A 97.691259
Ccucible
Everything B
S0.083219
2.391969
49.705439
49. 694259
Initial mass crucible, lid, and unknown hydrate;
Mass of unknown hydrate A-B
1st heating mass crucible, lid, and unknown
2nd heating mass crucible, lid, and unknown
3rd heating mass.Crueible, lid, and unknown
4th heating mass.crucible, Hd, and unknow
Final mass of unknown desiccant
Mass of Hydrate-desicant
Mass of Water (g)
Mass%'water unknown hydrate
Observations after heating:
The Substance Stacted to Stick t.
into a soilde
Observations after sprinkling the dehydrated sample with water:
when poucing the water on Sub5tance) the unk
hydcate absorbed the water, and hardened,
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please make sure the answer has the correct number of significant digitsarrow_forwardA balloon has a volume of 64.8 and 3.94 moles. If the volume decreases to 21.2, what is the new amount of moles? Round your answer to 2 decimal places.arrow_forward591470&isprv=&drc3D0&qi=2475808&cfql=1&dnb3D08fro= How many moles of Al2(CO3)3 are there in 127 g of Al2(CO3)3 ? Round your answer to 2 decimals. Your Answer: Answer units D Add attachments to support your workarrow_forward
- Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,O). What mass of water is produced by the reaction of 4.25 g of oxygen gas? Round your answer to 3 significant digits. ?arrow_forwardCalculate the number of grams of CaCO3 present and convert to mg. (enter your answer with 3 significant figures)arrow_forwardPlease see imagearrow_forward
- Liquid octane (CH3(CH,) CH;) will react with gaseous oxygen (02) to produce gaseous carbon dioxide (Co,). сн) (H2O). Suppose 63. g of and gaseous water octane is mixed with 90.2 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to 3 significant digits.arrow_forward0.145 mol C2 H6 Express your answer using three significant figures. VC = mol Submit Request Answer Part C 4.66 mol C4H10 Express your answer using three significant figures. ? VC = molarrow_forwardGive detailed Solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY