
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
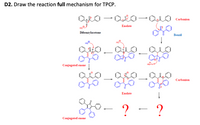
Transcribed Image Text:D2. Draw the reaction full mechanism for TPCP.
Carbanion
Enolate
HO
Dibenzylacetone
Benzil
Conjugated enone
но
Carbanion
Enolate
0-?
?
Conjugated enone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2) Draw the intermediates and the major organic product for each reaction sequence. 1) NaOCH₂ OCH3 2) H₂O, heat b. dild OEt 2) Ph 1) NaOEt (2 eq) OEt 3) H3O*, heatarrow_forward1. For each of the following reactions, draw in the curved arrow(s) required for the transformation and circle the type of chemical process at play. : NH2 O.. : NH2 a. i. Circle one: proton transfer nucleophilic substitution nucleophilic attack loss of a leaving group ..O ..O OCH3 OCH3 :15: b. i. Circle one: proton transfer nucleophilic substitution nucleophilic attack loss of a leaving group 11 :i: С. i. Circle one: proton transfer nucleophilic substitution nucleophilic attack loss of a leaving grouparrow_forwardDraw the products of each reaction.arrow_forward
- Which reaction intermediate (A or B) is more likely to form in the epoxide ring opening reaction? Reactions prefer to react through lower energy intermediates. Hint: how might you justify one structure being lower in energy than the other? Draw structures as part of your explanation why. Structure A Structure B ерoxide ring opening HO, : ОН Which alcohol structure shown below would be less acidic? Explain why. F F. Br Br Brarrow_forwardDraw the product of the reaction. 2 equiv. он H+arrow_forwardWhat reaction is occurring? Circle one. b) O: 1. LIAIH4. FHO- Nucleophilic Substitution Reduction 2. H20 Elimination Oxidation Mechanism:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY