
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Topic: Reactions of aromatic compounds
Draw a stepwise mechanism for the following reactions
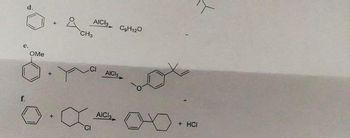
Transcribed Image Text:f.
d.
OMe
&
CH3
x
AICI3
CI
AICH
AICI
C₂H120
+ HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compare the basicity of piperidine and pyridine ?arrow_forwardProvide a plausible arrow pushing mechanism for the reaction below. 1 eq. H20 HO MEOH OMe cat. H-A OHarrow_forward3) Draw equations of the following reactions and and explain to which direction is the respective quillibrium shifted. a) cyclohexylamine + water b) aniline + sulphuric acid c) triethylamine + acetic acidarrow_forward
- 6) Answering the following questions about aryl amines a) Draw the curved arrow mechanism for the following reaction shown ( NH₂ CI NO₂ NINH, NO₂ b) Explain using diagrams why the following reaction does not work and why the above reaction in part a does work (1 NaNH, NO₂ NO₂ NH₂arrow_forwarda) Put these three common types of carbonyl compound in order of decreasing reactivity ester amide acid chloride b) For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable? c) For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O): Why makes this type of carbonyl so reactive to nucleophiles?arrow_forwardPlease don't provide handwritten solution..... what is the product for this substitution reaction belowarrow_forward
- Draw the organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as appropriate. Ignore any counterions. •H :F: :O: :O: H Na Ⓒ 0:0- Harrow_forwardArrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. Butanoic acid, Propionic acid, Propylamine, Butanearrow_forwardplease explainarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY