
Consider the reaction
Pure A is fed to a 1.0-dm3 CSTR where it reacts to form a desired product (D), which can then react further to produce an undesired product (U); both reactions are elementary and irreversible, and everything is liquid phase. The entering concentration of A is 1 mole/dm3 at a molar flow rate of 1 mol/min.
(a) Sketch the conversion of A, X, the instantaneous selectivity of D to U, SD/U, and the instantaneous yield of D, YD, as a function of space time (make sure to label them on the plot). You may want to write a sentence or two of reasoning for partial credit purposes.
(b) If at τ = 1.0 minutes the instantaneous selectivity, SD/U, is (1/2) and the conversion of A is (0.5),what are the specific reactions rates k1 and k2?
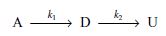
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 10 images

- 4. Ethylene oxide (C2H4O) can be produced by the partial oxidation of ethylene (C2H4) over a catalyst. An undesired side reaction is the complete oxidation of ethylene to CO2 and H2O. (R1) (R2) 2C2H4 + O2 → 2C2H4O C2H4 + 302 2CO2 + 2H2O The reactor is fed at 100 mol/hr of ethylene, and the molar feed rate of C2H4:O2 is 2:1. Air (79% N2 and 21% O2) is fed as the source of oxygen. 45% of the C2H4 is consumed in the reactor, and all of the O2 is consumed. What is the feed composition of all components in mol/hr? What are 31 and 32, the extents of reaction for the system, and the reactor effluent composition of all components in mol/hr.arrow_forwardFor the reactionFe3O4(s) + 4H2(g)3Fe(s) + 4H2O(g)H° = 151.2 kJ and S° = 169.4 J/KThe standard free energy change for the reaction of 1.55 moles of Fe3O4(s) at 270 K, 1 atm would be kJ.This reaction is (reactant, product) fill in the blank 2 favored under standard conditions at 270 K.Assume that H° and S° are independent of temperature.arrow_forwardFor the electrolysis of CuCl2 (molten) to form Cu(s) and Cl2(g):E°Cu2+/Cu = 0.339 VE°Cl-/Cl2 = 1.360 V What minimum voltage must be used to carry out the reaction? If 1.5V is used, how much electrical energy (in kJ) will be used to produce 2g of Cl2(s)?arrow_forward
- The process is to be designed for an 80% overall conversion of propane. The reaction products are separated into two streams: the first, which contains Hz gas, propylene (C3H6), and 0.5% of the propane that leaves the reactor, is taken off as product; the second stream, which contains the balance of the unreacted propane, 7% of the propylene in the product stream, and no H3, is recycled to the reactor. Calculate the flow rates and compositions of all streams and the single pass conversion of propane in the reactor.arrow_forwardUsing the tables in the pictures: Cyclobutane decomposes to ethylene according to the equation: C4H8(g) --> 2C2H4(g) Determine the order of the reaction and the rate constant, with units, based on the following concentration data. (Data in pictures)arrow_forwardQ1): Tw~362 K; Tn~342 Karrow_forward
- The decomposition of formic acid vapor according to the following reaction at 550oC follows a first-order kinetics, and at the above temperature, the reaction has a half-life of 240 s. HCO2H (g) ® CO2(g) + H2(g); Rate = k[HCO2H] If the initial concentration of HCO2H was 0.160 M, what is its concentration after four half-lives? (A) 0.010 M (B) 0.020 M (C) 0.040 M (D) 0.0050 Marrow_forward2. A 30.0-g sample of magnesium metal reacts with aqueous solution of hydrochloric acid at 25 degrees Celsius. From this, answer the succeeding questions. a) How much work (in Kilojoules) is done if the reaction is carried out in a closed vessel of fixed volume? Write answer in three significant figures. b) How much work (in Kilojoules) is done if the reaction is carried out in an open beaker? Write answer in three significant figures.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





