
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The existence of the NIH shift was established by determining the major product obtained from rearrangement of the following arene oxide, in which a hydrogen has been replaced by a deuterium.
a. What would be the major product if the NIH shift occurs? (Hint: A C¬H bond is easier to break than a C¬D bond.)
b. What would be the major product if the carbocation forms phenol by losing H+ or D+, rather than by going through the NIH shift?
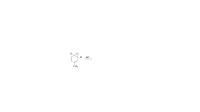
Transcribed Image Text:D
H-
HB+
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major organic product for the reaction of 1-phenylpropan-1-one with sodium hydride followed by ethyl iodide. Ignore stereochemistry. In Part 2 answer the question about the transformationarrow_forward3. Provide the major organic product of the following EAS Reaction. Think about what reaction is happening (look at the reagents). Think about which ring is more reactive (activating group v deactivating group). Do the EAS making sure the group being addition goes in the correct position on the correct ring. HO -SO₂H HNO₂, H₂SOarrow_forward2. Propose a reasonable mechanism for the following reactions. Label each reagent's role in the reaction. Assume aqueous work up to obtain neutral products. b. Ar- HO OH Ar ZnCl₂ Ar Hint: ZnCl₂ is a Lewis Acid.arrow_forward
- 4. Rhodamine B is useful dye prepared in a manner very similar to fluorescein. Due to the nitrogen donating groups, rhodamine B absorbs 550 nm light and fluoresces 580 nm light. a. What color would you expect for a rhodamine B solution? b. What is the color of the fluoresced (emitted light)? c. Provide a detailed mechanism for the formation of Rhodamine B from m- (diethylamino)phenol and phthalic anhydride in the presence of acid and heat. 2 НО. سوم m-(diethylamino)phenol phthalic anhydride H₂SO4 (cat.) Heat Rhodamine Barrow_forwardWhich statement best characterizes the following reaction of 2-phenylpropan-2-ol with HBr? OH CH3 CH3 + HBr Br CH3 CH3 + H₂O It is an SN1 reaction with bromide as the nucleophile and water as the leaving group. It is an SN1 reaction with HBr as the nucleophile and hydroxide ion (HO-) as the leaving group. It is a one step concerted reaction with bromide as the nucleophile and hydroxide ion (HO-) as the leaving group. It is an SN2 reaction with bromide as the nucleophile and water as the leaving group.arrow_forwardWrite a mechanism for the following reactionarrow_forward
- Where would the compound shown below undergo bromination with Bra/FeBr3? 6. -N-C ring 1 ring 2arrow_forward1. what is the Nature of the leaving group (LG)? 2. what is the relative size of activation energy (Ea) for each reaction? 3. what is the Hammond's postulate? 4. what are the Relative thermodynamic stability of the reactive intermediates? 5. what is Influence of the solvent (if given) on the reactions and intermediates?arrow_forward2. Provide the arrow pushing mechanism for the following reaction. Hint: it is a two-step mechanism. Br Br2 3. Draw the most likely product for each of the following reactions. а. Pd/C + D2 (one equivalent) b. + HFarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY