
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
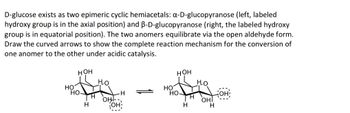
Transcribed Image Text:D-glucose exists as two epimeric cyclic hemiacetals: a-D-glucopyranose (left, labeled
hydroxy group is in the axial position) and B-D-glucopyranose (right, the labeled hydroxy
group is in equatorial position). The two anomers equilibrate via the open aldehyde form.
Draw the curved arrows to show the complete reaction mechanism for the conversion of
one anomer to the other under acidic catalysis.
HOH
HOH
Ho
HO
HO
HO
HO-
H
H
HO-
OH!--
H
H
OH
H
OHI
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- rivatives: Nucleophilic Acyl Substitution Reactions - EOC O C-O [References] One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehyde 3-phosphate. The process occurs by phosphorylation with ATP to give 1,3-bisphosphoglycerate, reaction with a thiol group on the enzyme to give an enzyme-bound thioester, and reduction with NADH. H-C-OH ATP ADP CH₂OPO32- 3-phosphoglycerate 0= OPO32- C H-C-OH CH₂OPO32 1,3-bisphosphoglycerate Enz-SH PO43- O= C S-Enz H-C-OH CH₂OPO3² (Enzyme-bound thioester) NADH/H+ O=C NAD*, Enz-SH H H-C-OH CH₂OPO3²- glyceraldehyde 3-phosphate Propose a structure for the first intermediate in the reaction of the enzyme-bound thioester with NADH to form glyceraldehyde 3-phosphate. To simplify the drawing process, substitute the structure below for the enzyme-bound thioester. S-CH3 substitute for the CH2CH3 enzyme-bound thioester You do not have to consider stereochemistry. • You do not have…arrow_forwardHow many stereoisomers are possible for an aldopentose?arrow_forwardDraw the two possible Haworth structures for each the following sugars in their cyclized form. Be sure to label each as an ? or a ? anomer.arrow_forward
- Classify each of the following compounds as reducing or non-reducing sugars. Please be very clear in your explanation.arrow_forwardDraw the hydrogen bonding of G-C and A-T pairs by hand. For each hydrogen bond, please point out which are hydrogen bond donors, and which are hydrogen bond acceptors.arrow_forwardUnderstanding monosaccharides (2-part problem) a) Draw both anomers of the 4C1 and 1C4 chairs of D-mannapyranose. Rank them from least stable to most stable, justifying your choice. b) Draw the acid-catalysed mechanism whereby D-fructose cyclizes to α-D-fructofuranosearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY