
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Can you help me with how to show the work? I have a hard time how to solve the problem. I know that the answer was 304K. I need to know how to convert 87 F to 31 C, then convert 31C to 304 Kelvin.
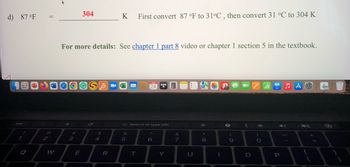
Transcribed Image Text:d) 87 °F
1
BL
Q
||
@
2
W
304
DOGS
3
For more details: See chapter 1 part 8 video or chapter 1 section 5 in the textbook.
GSC
E
$
4
K
R
%
M
5
First convert 87 °F to 31°C, then convert 31 °C to 304 K
G Search or type URL
T
24
*****
6
Y
&
7
☆
8
1
(
9
-
<
O
ZIA &
)
O
P
{
+
[
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-86 The specific heats of some elements at 25oC are as follows: aluminum = 0.215 cal/g · oC; carbon (graphite) = 0.170 caI/g oC; iron = 0.107 cal/g mercury = 0.033 1 caI/g oC. (a) Which element would require the smallest amount of heat to raise the temperature of 100 g of the element by 10oC? (b) If the same amount of heat needed to raise the temperature of 1 g of aluminum by 25oC were applied to 1 g of mercury, by how many degrees would its temperature be raised? (c) If a certain amount of heat is used to raise the temperature of 1.6 g of iron by 10oC, the temperature of 1 g of which element would also be raised by 10oC, using the same amount of heat?arrow_forward+ e Home ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001 Inbox-Outlook We... S Sugarpill Cosmetic... Shop | Origami Owl Wholesale Glass M... S The Corinthian Hou... zed Invitat... HW Provide Feedback P Pearson Contact Us Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions MacBook Air »)) DD DII O00 F12 F11 F10 F9 F8 F7 F4 F6 F5 F3 + $ & 3 0 9 4 7 8 6 5 } { P E U R Y D L K G F H T 2LOarrow_forwardpls help asap and Be sure your answer is rounded to the correct number of significiant digits.arrow_forward
- What are the temperatures Measurements for: M. N. O. P.arrow_forwardDoc X ← → C N Netflix Cos x Lapix M Inbc x X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537021_1?courseld=_19016_1&view=content The X Satu X Buy X a Ama X C Sho x X Gwha x o Ema X Intro X Cat X alculato "maxl ▸ (35 x ₁₁ G how x + F 8. A particular enzyme at a research facility is being studied by a group of graduate students. This enzyme has a Km value of 5.0 X 106 M. The students study this enzyme with an initial substrate concentration of 0.055 M. At one minute, 7 µM of product was made. What is the Vmax? 30arrow_forwardInbc X (531 X Con X I Bala X Ans X Inbc X CHE X 101 Che X С Ч-С х b My x Unk X Sea X E I ma X app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 7 of 40 Submit Which of the following compounds can form intermolecular hydrogen bonds? A) CH4 B) H2Se C) NH3 D) H2 E) All of these compounds can form hydrogen bonds. 10:36 AM e Type here to search 64°F 小 8/26/2021 (8)arrow_forward
- Docume x → C Netflix Thermo X Cosmet x C Laptops x M Inbox (7 X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content WOWER Saturati x C Buy TCL X a Amazon x C Show Y X G what's X Email - X យ ☆ b The dia x 90 h * ☐ 3. Calculate AG" and AG for the isomerization of dihydroxyacetone phosphate (DHAP) to glyceraldehyde 3-phosphate (GAP). This reaction takes place in glycolysis. At equilibrium, the ratio of GAP to DHAP is 0.0475 at 25°C (298 K) and pH 7. If the initial concentration of DHAP is 2 x 10 4M, and that of GAP is 2 x 10 M would the reaction be spontaneous under these conditions? (Answer: AG" = -RTINK= 7.54 kJ/mol, AG = -3.86 kJ/mol. Therefore, the reaction is spontaneous at the given concentrations) V :arrow_forwardbe TV COWLv2 | Online teaching and le... [Review Topics] Use the References to access import It is often necessary to do calculations using scientific notati each of the following calculations. 6.60 × 10-5) (9.20 × 10 105) =[ | 7.08 × 10¹ 5.09 × 10¹ (6.60 × 10-5) (5.09 × 10¹) 10-8) (7.08 × 104 1.00 x 10 104) Submit Answer Bb My Grades - Spring 20 Retry Entire Group 9 more group attempts remainarrow_forwardAt the beginning of the video, the students put a thermometer under a light. Then checked the temperature at the end. What evidence did you observe that light carries energy?arrow_forward
- Grad x I Cour X G 2.02: X G 2.0 m x G A hy X G oxide x G Exerc X E 4 -M X O Bana X G 1.84 x + college.com/course.html?courseld=16985674&OpenVellumHMAC=a0b841caaee7feddab1623a6c83a92c0#10001 I Review | Constants | Periodic MISSED THIS? Watch IWE 4.13; Read Section 4.10. You can click on the Review link to access the section in your e Text. 2.0 mol CH12 Express your answer in moles to two significant figures. Determine the number of moles of hydrogen atoms in each of the following samples. .? mol Submit Request Answer Part D 2.02 mol Cg H18 Express your answer in moles to three significant figures. mol Submit Request Answer P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy I Permissions | Contact Us I arch 3:46 88°F Mostly cloudy a a 4x 10/15/ 10 inort sc delete home end %24 4 & num 6 8 backspace lock Y U { [C home H J enter pause V B /| N M ↑ shiftarrow_forwardCH₂-COOH Citric acid has the formula HO-C-COOH I CH₂-COOH CH2–CDD - Na* + HD-C-CDD- Na CH₂-COO- COO-Na A 25.0 mL sample of a concentrated citrus fruit cordial component (e.g. for lime juice), used in the food & drinks industry, was diluted to 250 mL in a graduated volumetric flask. A 25.0 mL sample of this diluted solution, required, on average, 22.5 mL of a standard 0.100 molar sodium hydroxide solution using phenolphthalein indicator for the titration end-point. A) 0.0075 Assuming all the acid in the cordial was citric acid, calculate the concentration of the acid in g/mL in the original solution. B) 0.576 D and is tribasic acid, forming the tri-sodium salt on complete neutralisation with sodium hydroxide. 1.44 0.00225arrow_forwardCalculate the electrostatic force of repulsion for two protons separated by 93.0 pm. The charge of a proton is q = 1.6 x 10-1⁹ C. Express your answer to two significant figures and include the appropriate units. Fel = μA 2.7.10 6 Submit N Previous Answers Request Answer ? X Incorrect; Try Again; 5 attempts remaining Use the equation Fel = K(Q₁ Q₂/d²) to find the electrostatic force between the two particles. Q₁ and Q2 are the charges on the particles (charge of a proton here), and d is the distance of separation. The constant is given in the introduction and has the units Nm²/C². This means that the distance must be converted from picometers to meters using the relation 1 pm = 1 × 10¯ m. -12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning