
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
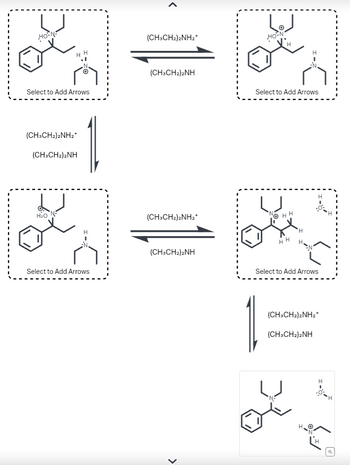
Transcribed Image Text:The diagram illustrates a mechanism involving the transformation of chemical structures in the presence of diethylamine (CH₃CH₂)₂NH and its protonated form (CH₃CH₂)₂NH₂⁺. The sequence includes a cyclic flow of possible reactions, where intermediates can be stabilized and transferred between different states.
1. **Initial Structures**:
- The top left and bottom left boxes contain a chemical compound with a benzene ring, and various substituents. Each structure includes a section labeled "Select to Add Arrows," indicating that there are potential electron movements or reaction pathways to explore.
2. **Reaction Arrows**:
- The arrows connecting these structures denote a reversible reaction between the compound and its interaction with diethylamine and its conjugate acid.
3. **Mechanism Steps**:
- At the top, the compound can transition between one depicted structure to another on the right through a step mediated by diethylamine. This transformation is indicated by arrow movement to the right.
- At the center, the reversible nature is shown again, highlighting the role of the protonated diethylamine in the backward reaction.
- Finally, at the bottom right, a transformation in the presence of diethylamine enables the compound to reach an end state, as depicted in the bottom right structure.
**Graphical Representation**:
- Each box contains chemical structures that indicate potential sites for reaction and electron movement.
- Chemical bonds and specific atoms are shown in standard chemical notation, with particular attention to nitrogen, oxygen, and hydrogen atoms due to their role in the reaction.
This diagram serves as an educational tool for exploring chemical reaction mechanisms and understanding the dynamic equilibrium between different chemical states in the presence of a basic amine and its conjugate acid.
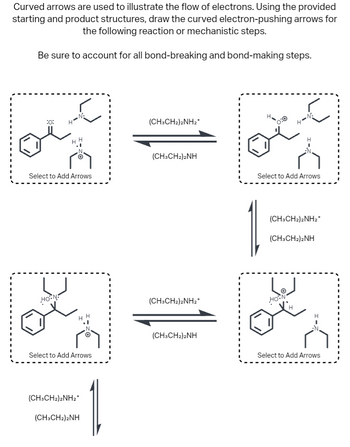
Transcribed Image Text:### Electron Flow in Reaction Mechanisms
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps.
Be sure to account for all bond-breaking and bond-making steps.
#### Diagrams
1. **Top Left Structure:**
- **Components:** This shows a benzene ring attached to a chain with an oxygen (O) and hydrogen (H) connected to a nitrogen (N) atom. There is a second nitrogen atom bonded to an ethyl group.
- **Label:** "Select to Add Arrows"
2. **Middle Structure:**
- **Components:** Represents the reaction pathway with two sets of arrows indicating forward and reverse reactions.
- **Label:** (CH₃CH₂)₂NH₂⁺ and (CH₃CH₂)₂NH
3. **Top Right Structure:**
- **Components:** Similar to the first structure with slightly different bonding, showing a positively charged nitrogen with electron pair interactions.
- **Label:** "Select to Add Arrows"
4. **Bottom Structures:**
- **Left and Right:** Represent the continuation of the reaction mechanism, showcasing similar components with small variations in bonding and labeling the forward (CH₃CH₂)₂NH₂⁺ and reverse (CH₃CH₂)₂NH reactions.
- **Label:** "Select to Add Arrows"
#### Notes:
- These structures depict the various stages in an electron-flow mechanism, emphasizing the role of curved arrows to show the movement of electron pairs.
- The arrows and chemical structures illustrate the equilibrium and dynamic nature of the reaction.
- Students should focus on identifying where electrons move during bond formation and cleavage.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- #1arrow_forward3. For the following synthetic schemes, please draw the product(s) to the right of the arrow. 1. Mg(0) 2. CO2 3. H30* Br 1. Mg(0) 2. CO2 3. H3O* Br MeO 1. (CH3)2CuLi 2. H20arrow_forwardThe following Wittig reagent can be synthesized by the synthetic sequence below. In the boxes, draw the reactants that are necessary for each reaction. Be sure to add nonzero formal charges to all atoms.arrow_forward
- Provide a complete mechanism for both steps in the reaction, including all lone pairs, formal charges, and curved arrows.arrow_forward% Leaving Group, Tertiary Sketch and Submit Above Carbocation, NOS neutral nucleophile Addition (Sn1), Mono- Substituted Proton Transfer, Deprotonation, Base (conjugate acid) Carrier H₂C H₂C H,C-C H CH₂ CH₂ CH₂ CH₂ a OH CH₂ H3C CH3 Leaving Group, Tertiary + Br: H3C-C CH3 OH CH, Carbocation, NOS neutral nucleophile Addition (Sn1), Mono-Substituted CH₂ CH₂ Proton Transfer, Deprotonation, Base (conjugate acid) Carrier OH H₂C CH₂ Sketch and Submit Above xaxaarrow_forwardHELP MEarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY