
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
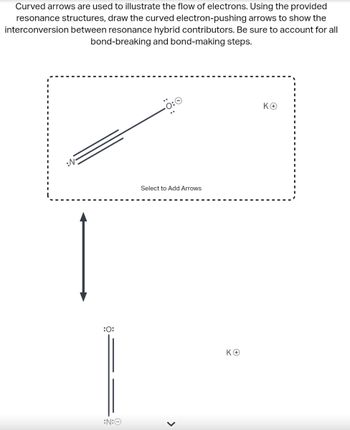
Transcribed Image Text:Curved arrows are used to illustrate the flow of electrons. Using the provided
resonance structures, draw the curved electron-pushing arrows to show the
interconversion between resonance hybrid contributors. Be sure to account for all
bond-breaking and bond-making steps.
:0:
:N:
Select to Add Arrows
>
ΚΘ
KO
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12. Provide one other contributing resonance form for structures A, B, and C. Show all electron movement using curved arrows. Between the two structures, circle the higher contributing resonance form. A В C HO.arrow_forwardHelp me complete the boxesarrow_forwardDraw curved arrows to show the movement of the electrons as the bond breaks.arrow_forward
- 15. Draw all the important resonance structures for the following ion, and show all the lone pair of electrons and formal charges. Show the electron flow by using arOws. NH NH 16. Draw skeletal structures of compounds accordinghto he rules provided and write their common names 100 16b. drawskeletal 16c. draw skeletal 16d drawskeletal arawskeletal an ether that contains abenzer ing (No limit on the total rumber of carbons) a secondary alkyl chloride with 4 carbons total a primary alcohol with 4 carbons total a tertiary amine with 6 carbons total common name common name common name common namearrow_forwardStep 3: Draw a curved arow to show formation of an oxygen-aluminum bond shown in the next panel. H C: + : 0: : 0: + + азаarrow_forward1. The following resonance structure are insignificant or incorrect. Explain resonance structures. alt-del 12-2 a. b. C. NH + NH 최arrow_forward
- it won't let me do OH togetherarrow_forward12. Provide one other contributing resonance form for structures A, B, and C. Show all electron movement using curved arrows. Between the two structures, circle the higher contributing resonance form. A B C ОНarrow_forward9. Draw all resonance structures of the following compounds. Show electron-pushing arrows.arrow_forward
- In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. σ: Ni) - F() Lone pair in sp orbital 1L o : N(p) – F(sp³) : N(sp³) – F(p) Lone pair in p orbital T: N(p) – F(p) Lone pair in s orbital σ: Nsp') - Ffp)arrow_forwardProvide the following 2 resonance structures and indicate whether or not they're major or minorarrow_forwardHandwritten solutions are strictly prohibitedarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY