
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Done
A education.wiley.com
AA
e Chapter 3
8 of 20
>
-/ 1
View Policies
Current Attempt in Progress
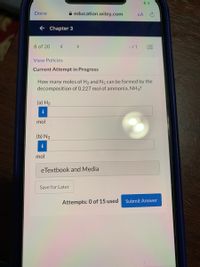
How many moles of H2 and N2 can be formed by the
decomposition of 0.227 mol of ammonia, NH3?
(a) H2
mol
(b) N2
i
mol
eTextbook and Media
Save for Later
Attempts: 0 of 15 used
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provid Part D How many molecules (not moles) of NH3 are produced from 1.02x10-4 g of H₂? Express your answer numerically as the number of molecules. ►View Available Hint(s) [5] ΑΣΦ 3.07 1020 Submit ● Previous Answers wwwwww.. ? molecules X Incorrect; Try Again; One attempt remainingarrow_forwardWhich of the following chemical formulas and names of compounds correctly matched? (A) PbO2 = lead dioxide %3D (B) H&NO3 = mercury(1) nitrate %3D (C) NazPO4 = trisodium phosphate (D) BaO2 = barium peroxide %3Darrow_forwardI just need help setting these up but 1 2 and 3 mostlyarrow_forward
- soul2.hkuspace.hku.hk My Questions | bartleby https://soul2.hkuspace.hku.hk/pluginfile.php/4896177/mod_resource/content/4/HPH.. Inbox (4,444) - manhin1127@gmail.com - Gmail 11) The equation for the reaction is: H.SO,(aq) + 2 NaОН(аq) —Na,SO,(aq) + H,0(1) Calculate the concentration of the sulphuric acid. 3. You are given the following half equations: → NO, + H2O so3 + H,O → so,² + 2H* + 2e HNO3 + H* + e° (a) Does HNO3 undergo oxidation or reduction in the above half equation? Explain your answer. (b) (i) Write a balance equation for the reaction between HNO; and SO;. (ii) Give the expected observation in the above reaction.arrow_forwardRemaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardQuestion 3 of 15 Submit How many moles of H2SO. are required to completely react with 7.20 mol of Al according to the balanced chemical reaction: Al(s) + 3 H:SO(aq) → Al:(SO.):(aq) + 3 H2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 6.022 x 1023 7.20 3.60 342.14 2.02 21.6 98.08 2.40 26.98 2 10.8 1 g H2SO, g Al:(SO.)s mol Al:(SO.)s g H2 mol H2SO4 mol Al mol H2 g Alarrow_forward
- JUNE AY 29 an active ingredient of Supp lements is iron II sulphate· One főm iron (a) what is nmass of %Fe in this compourol 30 NDAYarrow_forwardNAME: Hydrate Molar Mass of Anhydrous salt Molar Mass of nH₂O Molar Mass of Hydrate Percent Water in the Hydrate Ei 13 / 15 11 BaCl2 2 H₂O INSTRUCTOR: LAB INVESTIGATION 2 PROPOSAL Before you begin you will need to design an experiment for each unknown that includes alternative hypotheses, the test, and predictions. Complete the table and flow chart below. 208.23 g கேll 36.04 8 100% I CuSO4 5 H₂O 159.61 g MAKC "}}" ASUS VivoBook DATE: O SEC TION/ GROUP: CaCl2 2 H₂O 110.98 g ZnSO. • 7 H₂O 161.42 garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY