
Calculus: Early Transcendentals
8th Edition
ISBN: 9781285741550
Author: James Stewart
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
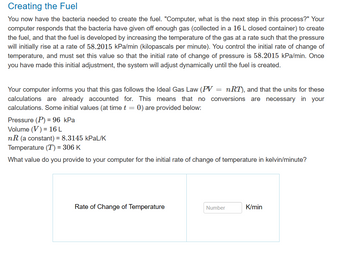
Transcribed Image Text:Creating the Fuel
You now have the bacteria needed to create the fuel. "Computer, what is the next step in this process?" Your
computer responds that the bacteria have given off enough gas (collected in a 16 L closed container) to create
the fuel, and that the fuel is developed by increasing the temperature of the gas at a rate such that the pressure
will initially rise at a rate of 58.2015 kPa/min (kilopascals per minute). You control the initial rate of change of
temperature, and must set this value so that the initial rate of change of pressure is 58.2015 kPa/min. Once
you have made this initial adjustment, the system will adjust dynamically until the fuel is created.
Your computer informs you that this gas follows the Ideal Gas Law (PV: nRT), and that the units for these
calculations are already accounted for. This means that no conversions are necessary in your
calculations. Some initial values (at time t = 0) are provided below:
=
Pressure (P) = 96 kPa
Volume (V) = 16 L
nR (a constant) = 8.3145 kPaL/K
Temperature (T) = 306 K
What value do you provide to your computer for the initial rate of change of temperature in kelvin/minute?
Rate of Change of Temperature
Number
K/min
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- MO22. Supercavitation is a propulsion technology for undersea vehicles that can greatly increase their speed. It occurs above approximately 50 meters per second, when the pressure drops off sufficiently to allow the water to dissociate into water vapor forming a gas bubble behind the vehicle. When the gas bubble completely encloses the vehicle, supercavitation is said to occur. Eight (n = 8) tests were conducted on a scale model of an undersea vehicle in a towing basin with the average observed speed x = 102.2 meters per second. Assume that speed is normally distributed with o = 4 meters per second. Use a = 0.05, test Ho: H = 100 versus H1 : µ < 100. What is the value of zo (2 decimal places, 0.00) and what can be concluded? Provide all the necessary solutions. z0 = Blank 1 and Blank 2 the Blank 3 hypothesis. Blank 1 Add your answer Blank 2 Add your answer Blank 3 Add your answerarrow_forwardA brine solution of salt flows at a constant rate of 5 L/min into a large tank that initially held 100 L of brine solution in which was dissolved 0.15 kg of salt. The solution inside the tank is kept well stirred and flows out of the tank at the same rate. If the concentration of salt in the brine entering the tank is 0.03 kg/L, determine the mass of salt in the tank after t min. When will the concentration of salt in the tank reach 0.01 kg/L? Determine the mass of salt in the tank after t min. mass= kgarrow_forwardA brine solution of salt flows at a constant rate of 8 L/min into a large tank that initially held 100 L of brine solution in which was dissolved 0.1 kg of salt. The solution inside the tank is kept well stirred and flows out of the tank at the same rate. If the concentration of salt in the brine entering the tank is 0.02 kg/L, determine the mass of salt in the tank after t min. When will the concentration of salt in the tank reach 0.01 kg/L? Determine the mass of salt in the tank after t min. mass = kg When will the concentration of salt in the tank reach 0.01 kg/L? The concentration of salt in the tank will reach 0.01 kg/L after minutes. (Round to two decimal places as needed.)arrow_forward
Recommended textbooks for you
 Calculus: Early TranscendentalsCalculusISBN:9781285741550Author:James StewartPublisher:Cengage Learning
Calculus: Early TranscendentalsCalculusISBN:9781285741550Author:James StewartPublisher:Cengage Learning Thomas' Calculus (14th Edition)CalculusISBN:9780134438986Author:Joel R. Hass, Christopher E. Heil, Maurice D. WeirPublisher:PEARSON
Thomas' Calculus (14th Edition)CalculusISBN:9780134438986Author:Joel R. Hass, Christopher E. Heil, Maurice D. WeirPublisher:PEARSON Calculus: Early Transcendentals (3rd Edition)CalculusISBN:9780134763644Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric SchulzPublisher:PEARSON
Calculus: Early Transcendentals (3rd Edition)CalculusISBN:9780134763644Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric SchulzPublisher:PEARSON Calculus: Early TranscendentalsCalculusISBN:9781319050740Author:Jon Rogawski, Colin Adams, Robert FranzosaPublisher:W. H. Freeman
Calculus: Early TranscendentalsCalculusISBN:9781319050740Author:Jon Rogawski, Colin Adams, Robert FranzosaPublisher:W. H. Freeman
 Calculus: Early Transcendental FunctionsCalculusISBN:9781337552516Author:Ron Larson, Bruce H. EdwardsPublisher:Cengage Learning
Calculus: Early Transcendental FunctionsCalculusISBN:9781337552516Author:Ron Larson, Bruce H. EdwardsPublisher:Cengage Learning

Calculus: Early Transcendentals
Calculus
ISBN:9781285741550
Author:James Stewart
Publisher:Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:9780134438986
Author:Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:9780134763644
Author:William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:9781319050740
Author:Jon Rogawski, Colin Adams, Robert Franzosa
Publisher:W. H. Freeman


Calculus: Early Transcendental Functions
Calculus
ISBN:9781337552516
Author:Ron Larson, Bruce H. Edwards
Publisher:Cengage Learning