
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
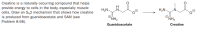
Transcribed Image Text:Creatine is a naturally occurring compound that helps
provide energy to cells in the body, especially muscle
cells. Draw an S2 mechanism that shows how creatine
is produced from guanidoacetate and SAM (see
Problem 8.66).
H2N-
H2N
NH2
NH2
Guanidoacetate
Creatine
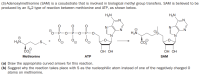
Transcribed Image Text:(S)-Adenosylmethionine (SAM) is a cosubstrate that is involved in biological methyl group transfers. SAM is believed to be
produced by an Sy2 type of reaction between methionine and ATP, as shown below.
H,N
H,N
N:
N
N
H3N.
H3N
CO2
ОН ОН
ОН ОН
Methionine
ATP
SAM
(a) Draw the appropriate curved arrows for this reaction.
(b) Suggest why the reaction takes place with S as the nucleophilic atom instead of one of the negatively charged O
atoms on methionine.
0=P-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Determine whether each of the following sugars is reducing or non-reducing.arrow_forwardIdentify the type of glycosidic bond for each of the following sugars. Choose from a-(1-2), a- (1-3), a-(1-4), a-(1-5), a-(1-6), B-(1-2), ß-(1-3), ß-(1-4), ẞ-(1-5), ß-(1-6), or a,ẞ- (1+2) CH₂OH 0 HO H H OH H H H OH O a-(1-6) glycosidic bond OB-(1-5) glycosidic bond OB-(1-6) glycosidic bond Oa-(1-5) glycosidic bond CH₂ HO H H OH H H 0 H HP OHarrow_forwardwhat is the drawn out electron pushing mechanismarrow_forward
- Classify each of the following compounds as reducing or non-reducing sugars. Please be very clear in your explanation.arrow_forwardDraw the complete electron pushing mechanism for the following transformation.arrow_forwardThe sugars can be classified as either aldoses or ketoses. Classify each structure with the correct name. НО НО H Н CHO —H -H -OH -OH CH₂OH НО НО Н CH₂OH c=0 H -н -OH CH₂OH НО НО н CHO ketose -H -Н OH CH₂OH Answer Bank aldose CHO H-C-OH CH₂OH НО Н CH₂OH c=0 -Н -OH CH₂OH " CH2OH O CH2OHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY