
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
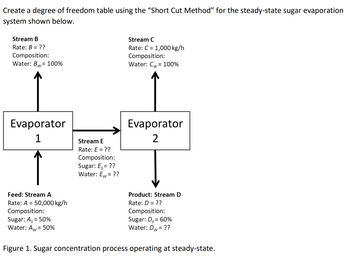
Transcribed Image Text:Create a degree of freedom table using the "Short Cut Method" for the steady-state sugar evaporation
system shown below.
Stream B
Rate: B = ??
Composition:
Water: Bw = 100%
Evaporator
1
Feed: Stream A
Rate: A = 50,000 kg/h
Composition:
Sugar: A, = 50%
Water: Aw = 50%
Stream E
Rate: E = ??
Composition:
Sugar: Es = ??
Water: Ew= ??
Stream C
Rate: C = 1,000 kg/h
Composition:
Water: Cw= 100%
Evaporator
2
Product: Stream D
Rate: D = ??
Composition:
Sugar: Ds = 60%
Water: Dw= ??
Figure 1. Sugar concentration process operating at steady-state.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- with degree of freedom analysisarrow_forwardLiza is a chemical engineer working for a wastewater treatment facility. One of thebyproducts of sewage treatment is sludge. After microorganisms, grow in the activatedsludge process to remove nutrients and organic material, a substantial amount of wet sludge is produced. This sludge needs to be dewatered, one of the most expensive parts of mosttreatment plant operations. Liza suggests that the sludge be dried and sold as fertilizer. She also mentions that burning the sludge as fuel is also effective. To burn this, fuel oil is mixed with it, and the mixture is burned in a furnace with air. Her boss agrees with burning but further data is needed in order to proceed with the plan. Since additional cost is required for the fuel oil, Liza needs to determine if burning is worth it. The data below shows the analysis for the sludge and the combustion products Sludge (%) Combustion Products (%) S - 32 SO2 - 1.52C - 40 CO2 - 10.14H2 - 4…arrow_forwardA. Graph Analysis: Cooling Curvearrow_forward
- Chemical Engineering A flotation cell is fed with solids at the rate of 250tph with a pulp having a density of 1.35. Material solid density is 3.2. The concentrate contains 15% of the feed solids mass and it has a pulp density of 1.15. The concentrate solid density is 3.5. How much water must be added to the tails to give an L/S ratio of 1.25. Give your assumptions and continue answering.arrow_forwardSarah mentions that burning the sludge as fuel is effective. To burn this, fuel oil is mixed with it, and the mixture is burned in a furnace with air. Her boss agrees with burning but further data is needed in order to proceed with the plan. Since additional cost is required for the fuel oil, Sarah needs to determine if burning is worth it. The data below shows the analysis for the sludge and the combustion products. Determine the weight percentage of carbon and hydrogen in the fuel oil to be used in order to produce a combustion products analysis, shown above, that is acceptable in DENR regulations. To be able to determine the additional cost needed for the fuel oil, how much dry sludge needs to be burned for every pound of fuel oil in the mixture?arrow_forward19B.7 Time for a droplet to evaporate. A droplet of pure A of initial radius R is suspended in a large body of motionless gas B. The concentration of A in the infinite distance from the droplet. gas phase is x, AR at r = R and zero at an (a) Assuming that R is constant, show that at steady state AB 2 dx dr R²NArlr=R (19B.7-1) XA where Nl-R is the molar flux in the r direction at the droplet surface, c is the total molar concentration in the gas phase, and DAR is the diffusivity in the gas phase. Assume constant temperature and pressure throughout. Show that integration of Eq. 19B.7-1 from the droplet surface to infinity gives АВ RNArlr=R = = -cD АВ In(1 - XAR) (19B.7-2) (b) We now let the droplet radius R be a function of time, and treat the problem as a quasi-steady one. Then the rate of decrease of moles of A within the drop can be equated to the instantaneous rate of loss of mass across the liquid-gas interface d -TR°C) = 47R°N olar = -4rRcDg In(1 – XXAR) (L) AR°C' In(1 - Х AR)…arrow_forward
- Please refer to the photo attached below. It has the questions:)arrow_forwardI need the answer as soon as possiblearrow_forwardExample Problems (Problems 3.3 and 3.14 of BK Dutta) Problem 3.3: A 0.8 cm diameter bubble of pure CO2 is injected into an excess well-stirred liquid at 25 C. The bubble diameter shrinks to 0.2 cm after 80 s. Calculate the average value of the mass transfer coefficient KL and Kx. How long will it take for the bubble to vanish if the mass transfer coefficient remains constant? The following data are available: (a) Total = 1 atm (b) solubility of CO2 in.water = 1.45 x 103 mole fraction (c) diffusivity of pressure CO2 in water=1.9 x 10S cm/sarrow_forward
- A water cleanup operation involves stripping vinyl chloride from contaminated ground water at 25 °C and 850 mm. Hg using a countercurrent, staged stripper. The feed has 5.0 ppm(molar) vinyl chloride, and the outlet water contains 0.1 ppm (molar) vinyl chloride. Inlet air used for stripping is pure. For a liquid flow rate of L = 1 kmol/h , determine the following : Minimum gas flow rate in kmol/h. If the actual gas flow rate = 2.0 × (minimum gas flow rate) calculate the number of stages Henry’s constant (HB) for vinyl chloride = 1243. 84 atm.arrow_forwardPlease help with homework problem.arrow_forwardThe feed supplied into the continuous distillation column at boiling point condition then the value ofarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The