
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
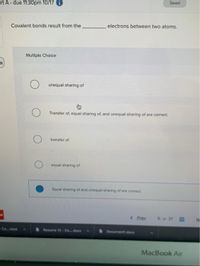
Transcribed Image Text:Saved
art A- due 11:30pm 10/17
Covalent bonds result from the
electrons between two atoms.
Multiple Choice
unequal sharing of
Transfer of, equal sharing of, and unequal sharing of are corect.
transfer of
equal sharing of
Equal sharing of and unequal shaning of are comect
( Prev
S of 37
Co. tml
Resume 12-C..docs
Documentt doCE
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- M Inbox (6) - danasia.... Advanced Material Predicting the relative lattice energy of binary ionic compounds In each row, pick the compound with the bigger lattice energy. Note: lattice energy is always greater than zero. Which compound has the bigger lattice energy? BaF O KBr Rb₂ S O Explanation 1o_u-IgNslkr7j8P3jH- 31 Ascension - Calend... Shared drives - Goo... & Intranet X Esc BaO 4 KCI SrS Check Type here to search Ś F1 J F2 10 F4 F5 0/ DE F6arrow_forward(T2 2021) ellbeing 2 Health COVID-19 18 onי Using the values provided in the table below, calculate AS for the following reaction 250 (g)-Olg) + 2SO;(g) yet wered Species S / J mol1 K1 ked out of SO-(g) SO (g) Olg) 248 114 Fla 205 estion Oa.473 J mo K- Ob929 J molK OC473J molK Od-339 J molK Oe 339 J mat Question 19 Balance the equation below for the reaction under acidc aquous condos Answer saved Marked out of What is cooffciont for the PRag Select onearrow_forward9:19 O ul LTE O Review ABookmark MISTRY HONORS 2021-227 of 21 NH3(g) + _ O2(g)→_ N2(g) + _ ,0(g) When the reaction above is completely balanced, the coefficient for NH, will be O A 4 OB 3 OC 5 OD 2 "3 "5 6 8 Q T Y G.arrow_forward
- E. There are 2 structures possible 0 ट्रarrow_forwardNernst equation for pair H3ASO4|HASO2: E = E° + -In- nF RT a(H,AsO,)a²(H*) a(HASO,) RT a(H,AsO,)a(H*) E=E° + -Ig nF a(HASO,) Inª(H3A$O,)a²(H*) a(НASO,) RT E =E° nF RT a(H,AsO,) -lg nF E = E° + a(HASO,)arrow_forwardAHCN + 50 2(g) 2H2OG) + 4CO2(9) + 2N2 Calculate AS using the table provided. Compound S re HCN HČN 94.1 205.0 H,0 189.0 214 192arrow_forward
- 4. The n-electron system in benzene may be approximated as a particle-on-a-ring with a radius of r, which is also the length of the C-C bonds in the ring. This bond length is approximately 1.39 A. The energy levels for the p-o-a-r are given by: n'h E, ,where n = 0, t1, ±2, 13, . 2m,r? The six r-electrons in benzene fill the (singly degenerate) n 0 level, and the (doubly degenerate) n=t1 level. The first excited state, then corresponds to exciting an electron from n=1 to n=2. a) Using the given bond length, calculate the energy change for the benzene molecule as an electron is excited from n=1 to n=2. b) Calculate the wavelength of light that benzene would need to absorb to undergo that energy change. Compare that with the experimental value of 268 nm. c) In what portion of the electromagnetic spectrum is this wavelength? d) Using the experimental wavelength from part b above, calculate the effective bond length in benzene. e) Is the particle-on-a-ring approximation valid? Give a…arrow_forwardSyllabus for SP2021 CH x My Home x OWLV2 | Online teachin x MindTap - Cengage Le: x Shopping Cart X. now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take え☆( Update L Dashboard we Login | BlazerlD Digital Learning &.. us Calculus Testbank -.. X Excel Do Desmos | Graphing... h Hulu S Solutions to Stewar... I Mechanisms Review Topics) References] r.. 1 pts 2req Use the References to access important values if needed for this queztion. ns.. 1 pts 2req The rearrangement of cyclopropane to propene at 500 °C (CH,),→CH;CH=CH, -1 is first order in (CH,), with a rate constant of 6.70x10 s. en... 1 pts 2req s have passed. If the initial concentration of (CH,), is 8.35×10- M. the concentration of (CH,), will be 1.73x10- M after ons.. 1 pts 2req fo 1 pts 2req Retry Entire Group 9 more group attempts remaining Submit Answer ons 1 pts 2req 1 pts 2req ms L. 1 pts 2req Previous Eerial Instructor Save and Exit 2:09 PM >国日后会 1/31/2021arrow_forward||| = O ELECTRONIC STRUCTURE AND CHEMI... Deducing valence electron configuration from trends in... The first five ionization energies (IE,through IE,) of a Period 2 element have the following pattern: kJ/mol ..... IE₁ E2 IE3 IE4 IE5 0/5 Make a reasonable quess about which element this is. Enter its chemical symbol Explanation Check Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Andrew V Privacy Center ? allo Ar Accessibilityarrow_forward
- My NSUI Northeastern State O X Screenshot 2020-11-27 at 7.20.5... O O Q Q Given the following information: Heat of sublimation of K(s) = 89 kJ/mol %3D Bond energy of HF = 565 kJ/mol Ionization energy of K(g) = 419 kJ/mol Electron affinity of F(g) = –328 kJ/mol "attice energy of KF(s) = –808 kJ/mol > Bond energy of H2 = 432 kJ/mol %3D Calculate the net change in energy for the following reaction: 2K(s) + 2HF(g) → 2KF(s) + H2 (g) Change in energy kJ Submit Answer Try Another Version 3 item attempts remaining O 7:21arrow_forward8.1929of Nickel lii suffate hydrate when heated lost all its water of hydration leaving behind H.8269 of the anhydrate The equation of the reaction is Heat Niso4 + xH20 Anhychrute Hydrate Calculate 9Thhe mass of the water of hydration in the hydrate exPelled by heating b The modes of the CThe 'molesof anhychrate Nison inthe H.826g water Leaves 95 vapor water of hydration dThe ration of the meoles of the water of hycration to he mules of the anhycrate e what is x in Niso4ixH20arrow_forward8:42 e ul LTE く https://testnavclient.psonsvc.net//question/37ae15ac-69b1-43c8-97b4-937c4ad3606e/d2839865-2121-479f-859c Review RBookmark CIENCE CHEMISTRY HONORS MP2 2021-22 / 2 of 21 The paticle diagram below represents a solid sample of silver. Which type of bonding is present when valence electrons move within the sample? O A Covalent bonding O B lonic bonding Oc Hydrogen bonding On Metallic.bondina O Type here to search 3. 4. 6. Q W T Y. Retake Use Photoarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning