
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question

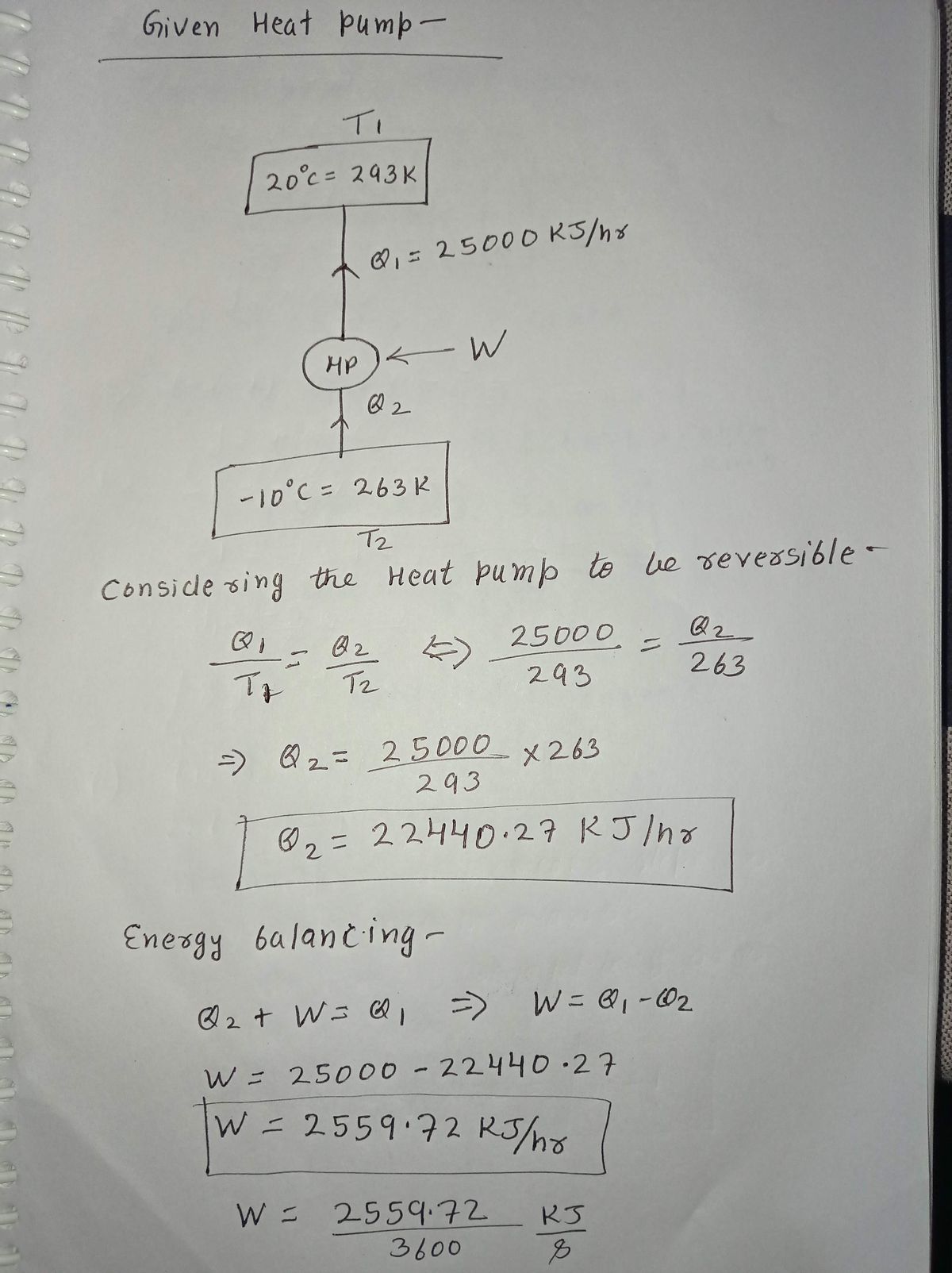
Transcribed Image Text:By supplying energy to a house at a rate of 25,000 kJ/hr, a heat pump maintains the
temperature of the dwelling at 20 C when the outside air is at -10 C. If electricity
costs 8 cents per kW-hr, determine the minimum theoretical operating cost to heat
the house for 24 hours.
$1.97
O $1.37
$1.75
O $1.51
O$1.64
Expert Solution
arrow_forward
Calculating work in Kw
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 6) A room’s sensible space load is from occupants and infiltration, but excluding intentional ventilation, is 3.2 kW. A total of 600 L/s of outside air is required. What is most nearly the room sensible heat ratio?arrow_forwardA gas furnace has an efficiency of 75%. How many BTU will itproduce from 1000 BTU of natural gas.arrow_forwardBased on the mechanisms of thermal energy exchange what would happen if you exercised in a colder place where it's 5⁰C.arrow_forward
- Task 1 Write down the energy balance equation from the first law of thermodynamics for a closed system and then put the equation for an open system. Explain the difference between the two equations.arrow_forwardIf we wanted to supply all the electricity needs for New York City (NYC), with a popula- tion of about 10 million people, using photovoltaics technology, how much land area would we need? What other elements would be required for such an idea to work for NYC? How large of an investment would this involve, and how would the price of electricity in ¢ per kWeh have to increase in order to recover that investment in 10 years at an interest rate of 8%? For comparison, the average New Yorker paid 12¢/kWeh in 2002.arrow_forward3. An automobile engine delivers 25hp to the driveshaft with the thermal efficiency of 30%. The fuel has a heat content of 40 000kJ/kg. Calculate the rate of fuel consumption (kg/hr) and the heat rejected (kW) through the radiator and exhaust.arrow_forward
- A heat pump with the COP of 3.8 supplies heat at the rate of 289 kJ/min. If a bar heater was used and the electricity cost R2.50 per kWh peak and 200 cents off-peak , how much would be saved using the heat pump for 7 days if the off-peak hours are from 22:00 hr to 6:00 hr for every day of the week. Provide the answers to 2 decimal places and insert the unit in Rands after the answerarrow_forwardb. The ceiling of a certain room in a student's dormitory has an area of 200 ft'. It contains insulation such that its R-value is 30. If the room is maintained at 22°C and the attic has a temperature of 50°C. How many Joules of heat flows through the ceiling into the room in 1 day?arrow_forward2. Carbon Dioxide with specific heat ratio of 1.299 is used as a working fluid in a piston-cylinder assembly which is designed handle fluid at constant temperature. The heat was applied to CO2 at 350ºF allowing an expansion in the piston cylinder assembly. If the heat loss accounts to 20Btu/lb, determine the work done by piston displacement (Btu/lb).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY