
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
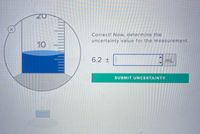
Transcribed Image Text:Correct! Now, determine the
uncertainty value for the measurement.
10
6.2 ±
mL
SUBMIT UNCERTAINTY
20
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- #10 dosage calc problemarrow_forward3. A student weighed an unknown mass directly on an electronic balance of intermediate sensitivity, and got a result of 28.774 g. The student then realized a mistake had been made, and weighed the same unknown by difference on the same balance with the following results:Mass of beaker plus unknown = 59.121 g.Mass of empty beaker = 30.346 g.When the sensitivity of the balance is taken into account, the two values obtained for the unknown mass are considered to be:a. Differentb. The SameExplain your answer.arrow_forwardI just want to check if I did the calculations with the measurements correctly and with the right amount of signiarrow_forward
- ARSE HE MAKING MON NCLEX ! O MEASUREMENT Setting up a one-step unit conversion A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. 46. B cm Explanation g m Check New appointment scheduling TXD ps Privers License social 7594 @gmail.com les su got this !!! 2 0.0 X H 00 5 DELL 2023 McGrawarrow_forwardHi I need help pleasearrow_forwardcompare the intial and final density if the gummy bear. Was the change in density influenced more by a change in mass or a change in columearrow_forward
- A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. (16. cm' ) 0= ? m ?m Applications | Amazon.jarrow_forwardA) 6 Q24).The accepted value is 15.63. Which correctly describes this student's experimental data? B) 10 A) Accurate but not precise B) Precise but not accurate Trial Measurement C) Both accurate and precise D) Neither accurate nor precise 12.84 2 13.02 3 12.96arrow_forward||| inds soon O MEASUREMENT AND MATTER Multiplication and division of measurements Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the table. proposed multiplication or division (6.0 g)-(7.0 g) = ? 2 200. mg 0.050 g = ? (7.1 dL) (0.70 L) = ? Explanation Check Is this possible? O yes O no O yes O no O yes O no result 0 0 0 x10 ロ・ロ X Q Search u μ 8 LO 0/5 4 O Jessica V olo Ar © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY