
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
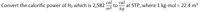
Transcribed Image Text:cal
Convert the calorific power of H2 which is 2,582
cal
to
m3
at STP, where 1 kg-mol = 22.4 m3
%3D
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If you combine 430.0 mL430.0 mL of water at 25.00 ∘C25.00 ∘C and 140.0 mL140.0 mL of water at 95.00 ∘C,95.00 ∘C, what is the final temperature of the mixture? Use 1.00 g/mL as the density of water.arrow_forwardHow much heat is released when 39.0 grams of ethanol (C2H6O, MW=46.07 g/mol) reacts according to the equation below: 2C2H6O + 7O2 = 2CO2 + 3H2O. DH= 2733.6 kJarrow_forwardIf you combine 230.0 mL230.0 mL of water at 25.00 ∘C25.00 ∘C and 140.0 mL140.0 mL of water at 95.00 ∘C,95.00 ∘C, what is the final temperature of the mixture? Use 1.00 g/mL as the density of water.arrow_forward
- A substance receives 1 kJ of pressure-volume work and produces 2 kJ of heat at constant pressure. The enthalpy of the substance (pick one):arrow_forwardNaHCO3 (aq) + CH3 COOH (aq) —> CO2 (g) + H2O (l) + CH3 COONa (aq) Calculates the amount of heat energy absorbed by the solution in the calorimeter or the amount of heat energy released by the reaction. Note: 100ml of CH3COOH, 2.5ml of NaHCO3, initial temp of 23c, final temp of 21carrow_forwardIf you combine 240.0 mL240.0 mL of water at 25.00 ∘C and 110.0 mL of water at 95.00 ∘C, what is the final temperature of the mixture? Use 1.00 g/mL as the density of water.arrow_forward
- At 1 bar, how much energy is required to heat 55.0 g of H2O(s) at −10.0 ∘C to H2O(g)at 173.0 ∘C?arrow_forwardUsing the equations determine the enthalpy for the reactionarrow_forwardWhen 56.8 of lead reacts with 3.50 L of oxygen gas, measured at 1.00 atm and 25.0 degrees Celsius, 60.1 kj of heat is released at constant pressure. What is the change in enthalpy at standard condition for the reaction (Please type answer no write by hend)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY