
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:9:25
Question 38.c of 40
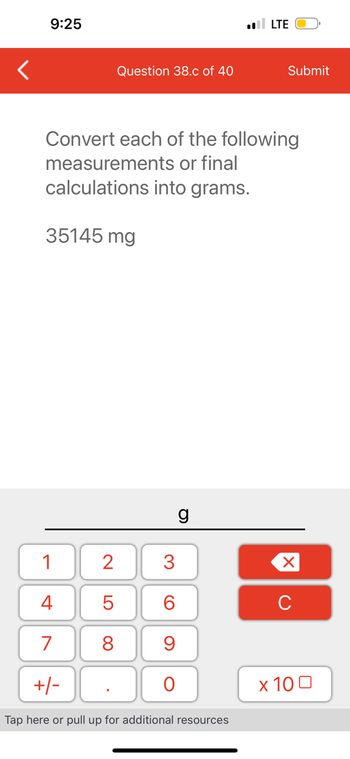
35145 mg
Convert each of the following
measurements or final
calculations into grams.
g
1
4
7
+/-
Tap here or pull up for additional resources
2 3
5
6
8
9
O
. LTE
Submit
XU
x 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A firkin = 40.82 kg (inexact). How many firkin in 76.2 kg? 3110 fir O 1.866 fir 1.87 fir 3.21x10-4 fir 0.5357 firarrow_forwardCalculate the number of grams of CaCO3 present and convert to mg. (enter your answer with 3 significant figures)arrow_forwardThe mass of a certain atom is 2.086 x 10°22 g. This is the same mass as 2.086 x 10-16 mg. 2.086 x 10-25 kg. 2.086 x 10-28 Hg. O 2.086 x 10-31 ng.arrow_forward
- 1.2×10−2 mol ethanol Express your answer using two significant figures.arrow_forward0.145 mol C2 H6 Express your answer using three significant figures. VC = mol Submit Request Answer Part C 4.66 mol C4H10 Express your answer using three significant figures. ? VC = molarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 298.6 m 87.8 s 20.94 mol L x 13. L = 508.5 mol 0.78 L m mol mol Xarrow_forward
- Based on a grade 11 chemistry student, answer the following question based on the provided table: Using the information contained in the Analysis of Percent Composition of Pills from Mary’s Mother’s purse and table 4, are you able to determine the identity of any of them?arrow_forwardBased on a grade 11 chemistry student, answer the following question based on the provided table: The question: Find the percent composition of carbon in acetaminophen, Aspirin, Ibuprofen and Naproxen.arrow_forwardDetermine the mass of CaSO4 in the sample based on the following data to two decimal places mass of beaker and cover 50.08 mass of beaker, cover, and sample before heating 51.06 mass of beaker, cover, and sample after heating 50.84arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY