
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
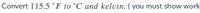
Transcribed Image Text:Convert 115.5 °F to °C and kelvin. ( you must show work,
Expert Solution
arrow_forward
Step 1
In the given question we have to convert the value of Fahrenheit into following units. i.e celcius (°C) and Kelvin (K)
we know that,
the given formula,
Converting from Fahrenheit to celcius
°C = (°F - 32 ) × (5/9)
Converting from Fahrenheit to Kelvin
K = (°F - 32 ) × (5/9) + 273.15
for 115.5 °F to celcius
by using the formula,
°C = (°F - 32 ) × (5/9)
= ( 115.5 - 32 ) × (5/9)
= 83.5 × (5/9) °C
= 417.5 / 9 °C
= 46.389 °C
for 115.5 °F to Kelvin
by using the above formula,
K = (°F - 32 ) × (5/9) + 273.15
= ( 115.5 - 32 ) × (5/9) + 273.15 K
= 83.5 × (5/9) + 273.15 K
= 46.389 + 273.15 K
= 319.54 K
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A spherical container is filled with a gas, what is the mass in grams of this gas if the density of it is 0.2201 and the diameter of the container is L 4.65 ft? You must use the following equality. You MUST show all work. *1 ft = 12 in 1 in = 2.54 cm 1000 mL = 1 L Vol of sphere = %3| .3arrow_forwardIn Ray Bradbury’s 1953 novelFahrenheit 451, 451°F is said to be the temperature at which books,which have been banned in the story, ignite. Convert 451°F to theCelsius scale.arrow_forwardThe temperature of 390 k is warmer than which one of the following temperatures? a- 109 °C b- 250 °F c- 135 °C d- 245 °Farrow_forward
- A 355 g sample of water goes 85 degrees C to 42 degrees C. Calculate the amount of heat energy (in calories) involved in this process. Report the answer in significant figures.arrow_forwardWhich of the following is a physical property? Which of the following items is a pure substance? What is the value of 98 °F in units of °C? Convert 68852 millijoules into Calories. (Write your answer in the decimal form? When table salt is placed in water and stirred it dissolves and a clear liquid is obtained. This process is a: Some groceries are placed in a deep freezer that is set at 18 oF. What is this temperature in Celsius? Thermal energy is: Which of the following statements is true about a compound? How many joules are there in a 255 Calorie snack bar? Which of the following is a physical property? If the monthly electricity consumption of a household is 6,619 kWh, what is this consumption expressed in kJ? Which of the following items is a chemical property? If you hold a solid piece of pure gallium metal in your hand, your body heat will melt the gallium into its liquid form. This illustrates which of the following? On a cold day the atmospheric…arrow_forwardGive typed full explanation not a single word hand written otherwise leave itarrow_forward
- plz answer both , if u plan to do only one then skip , do both plzarrow_forwardA block of aluminum is heated with 600J of energy. Its temperature increases from 10 degrees Celcius to 47 degrees Celcius. What is the mass of the block?arrow_forwardHow many kilocalories are lost when 580g of water cools from 67 degrees celsius to 22 degrees Celsius.?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY