
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
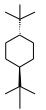
Considering the observed rotation value indicated in the table, and considering that the measurement of this rotation was
performed in a 4dm tube, from a 1g/ml solution, calculate: Show calculations
a-Specific rotation of this compound.
b-Rotation observed for a mixture in which the excess
enantiomer of the enantiomer of the substance of the utterance was 50%.
observed rotation: +40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This must be typed/digitally illustrated. It CANNOT be hand-drawn. (it will be marked as wrong if it is hand-drawn)arrow_forwardIdentifying Isomers from Fragmentation Patterns Which mass spectrum (A or B) corresponds to each isomer: 2methylpentane [(CH3)2CHCH2CH2CH3] or 2,2-dimethylbutane [(CH3)3CCH2CH3]?arrow_forwardThe following data was collected for this experiment: A sample of 0.8281 g of phenylsuccinic acid was dissolved in 10 mL of acetone. This sample gave a reading, aobs, of +10.278 deg on the polarimeter. A tube measuring 1 dm was used for the sample. Calculate the enantiomeric excess (EE) of the sample. The literature-specific rotation, [a]lit, of phenylsuccinic acid in acetone is 173 deg. Give your answer to 3 significant figures. Calculate the percent (%) of (+)-phenylsuccinic acid that is in this sample. Give your answer to 3 significant figures. Calculate the percent (%) of (-)-phenylsuccinic acid that is in this sample. Give your answer to 3 significant figures.arrow_forward
- Dr. VMV weighed out 1.02 g of menthol and dissolved it into 10.0 mL of absolute ethanol in a 10.0 mL volumetric flask. The optical rotation for this solution was obtained in a cell with a 50 mm optical path length, found to be -2.508°, using the Na D-line at 25.1 °C. What is the specific rotation?arrow_forwardYou dilute 1g of an orgainc liquid, with ethanol, to 10mL in a volumertic flask. This solution is analyzed using polarimerty (at 20oC), and an optical rotation of -6.8o is observed. Calculate the specific rotation of the oraginc liquid, if the sample cell that was used was 100mm.arrow_forwardDraw the structural formula that is consistent with each of the 13CNMR spectra shown belowarrow_forward
- Calculate the degree of unsaturation. Assign the relevant peaks in the IR spectrum. Assign the peaks in the 13C NMR spectrum. Assign the peaks in the 1H NMR spectrum.arrow_forwardGiven the HNMR provided, give the structure of the molecule. The molecular formula is C3H₂O3. The IR shows strong peaks at 3300 cm³¹ and 1720 cm ¹. Note that the chemical shift axis is not to scale: there is a large gap between 6-11 ppm and a gap at 1 ppm. 12 1H 11 6 1H 5 Chemical Shift (ppm) 3 2H 2H 2 TMSarrow_forwardCalculate the HDI, propose functional groups, analyze each spectral data and assign a structure to the compound with a molecular formula C6HgCl₂O₂ if the two chlorines are in the geminal position. IR 1739 cm ¹ C-13-normal 170 63 53 35 31 18 DEPT-135 0 0 + 0 - + 3 DEPT-90 0 0 0 0 0 0 Conclusionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY