
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
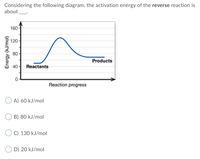
Transcribed Image Text:Considering the following diagram, the activation energy of the reverse reaction is
about
160
120
80-
Products
40
Reactants
Reaction progress
A) 60 kJ/mol
B) 80 kJ/mol
C) 130 kJ/mol
D) 20 kJ/mol
Energy (kJ/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- All other factors held constant, which graph represents the sample/reaction with the largest value of k? The vertical line in each case represents the activation energy for the reaction.arrow_forwardAt equilibrium, the mechanism 2 The reaction, A + 3B-> D + F was studied and the following mechanism was established: A + B -> C (fast) C + B-> D+E (slow) E + B > F (very fast) W #3 Peaction rate equals the reverse reaction rate. E $ 4 R % 5 T MacBook Pro 6 Y & 7 The species E is properly described as a co-factor an intermediate a reactant an enzyme a catalyst The species C is properly described as a catalyst an enzyme an intermediate a co-factor a reactant U 8 ( n Oarrow_forwardWhich of the following will decrease the activation energy, Ea, of a reaction? O Increasing the reactant concentrations Removing the products as they form O Increasing the temperature O Adding a catalyst O Decreasing the pressure A Moving to another question will save this response. M esc 20 F3 F1 F2 F4 F5 @ #3 24 4 Q W R. Aarrow_forward
- Potential Energy (kJ/mol) 112.5- 90.0- 67.5- 45.0- 22.5. 0.0 1 Which statement is incorrect for the following reaction profile for a single collision reaction? Reaction Profile 3 A) Point 1 is the reactant. B) The activation energy is 90 kJ/mol. C) The activated complex exists at point 2. D) AH = 0.0 kJ/mol. E) The reaction is endothermic.arrow_forward7arrow_forwardUsing the potential energy diagram determine the activation energy (each division is 10kg).arrow_forward
- tab lock atrol D esc Question 14 Based on the initial-rate data below, which is the magnitude (units omitted) for the specific-rate constant for this reaction? Rate (M/s) 1.3 x 10-7 5.2 x 10-7 1.0 x 10-6 [HgCl2] (M) [C2042-] (M) 0.10 0.10 0.10 0.20 0.20 0.20 O 5.2 x 10-6 O 1.3 x 104 O 1.3 x 10-5 O 2.0 x 10-7 Question 15 ! An chemical reaction has specific-rate constant 1.10 x 104 s¹ at 470 C. How many hours are required for a 0.335 M solution to decrea to 0.051 M? 1 O A B Ā N @ 2 W S X 2 option command w # E D DS 4 C R TI % 5 V 2 T G > A 6 MacBook Pro CO B Y 4) & 7 H U N 00 8 J - M ( 9 K O ) O < H 5 L P commandarrow_forwardFor the reaction mechanism below answer the questions. Fast equilibrium OH- + O2NNH2 --> O2NNH- + H2O Slow O2NNH- --> N2O + OH- a. What is the overall reaction?arrow_forward3arrow_forward
- Energy (kJ/mol) 50 -37.5 25 +12.5 1 Which statement is incorrect for the following reaction profile? 2 Reaction Profile 3 A) The reaction is exothermic. B) The activation energy is 50 kJ/mol. C) AH = -25.0 kJ/mol. D) Point 2 is an activated complex. E) The products are lower in energy than the reactants.arrow_forwardNumber 2arrow_forwardPlease help me answer these 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY