
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
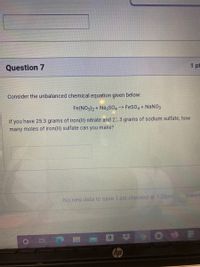
Transcribed Image Text:Question 7
1 pt
Consider the unbalanced chemical equation given below:
Fe(NO3)2 + Na2SO4 -> FeSO4 + NaNO3
If you have 25.3 grams of iron(II) nitrate and 25.3 grams of sodium sulfate, how
many moles of iron(II) sulfate can you make?
SUBMIT
No new data to save. Last checked at 1:28pm
hp
Expert Solution
arrow_forward
Step 1
The number of moles of FeSO4 produced is given below
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following equation: NH3(g) + O2(g)———> N2(g) + H2O(g)arrow_forwardNickel(II) chloride reacts with sodium phosphate to precipitate nickel(II) phosphate.3NiCl2(aq) + 2Na3PO4(aq) → Ni3(PO4)2(s) + 6NaCl(aq)How many moles of nickel(II) chloride are needed to produce 0.715 mol nickel(II)phosphate?arrow_forwardWhen heated, Fe(OH)3 decomposes into Fe2O3 and water, according to the following equation: 2 Fe(OH)3 (S) > Fe2O3 (s) + 3 H2O (g). If 24.24 g of Fe(OH)3 decomposes, and 13.56 g of Fe2O3 are obtained in the reaction, what is the percent yield of the reaction?arrow_forward
- Balance the following chemical reaction, be sure to include “1” in the blank for any compounds with a stoichiometric coefficient of 1. CaCl2(aq) + Li3PO4(aq) → LiCl(aq) + Ca3(PO4)2(s)arrow_forwardCalculate how many grams of O2(g) can be produced from heating 33.7 g KCIO,(s).arrow_forwardFor the chemical reaction 2KI+Pb(NO3)2⟶PbI2+2KNO32KI+Pb(NO3)2⟶PbI2+2KNO3 what mass of lead(II) iodide is produced from 2.072.07 mol of potassium iodide?arrow_forward
- In the following reaction, how many grams of lead(II) nitrate, Pb(NO3)2, will be needed to react with 60.7 g of potassium iodide, KI? 2KI+Pb(NO3)2 -----> PbI2+2KNO3arrow_forwardThe thermite reaction, once used for welding railroad rails, is often used for an exciting chemistry demonstration because it produces red-hot molten iron. The reaction is: Fe2O3(s) + 2Al(s) → 2Fe(I) + Al2O3(s)If you start with 50.0 g of iron(III) oxide and 25.0 g of aluminum, what is the limiting reagent? What is the maximum mass of aluminum oxide that could be produced? How much aluminum oxide would be produced if the yield is 93%?arrow_forwardIn the following reaction, which element is the limiting reactant? 2Fe(s) 3Cl2(g) → 2FeCl3(s) iron chlorine neither both Cannot be determined without the original starting mass of both reactants.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY