
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
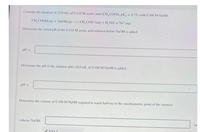
Transcribed Image Text:Consider the titration of 25.0 mL of 0.118 M acetic acid (CH, COOH, pK, = 4.75) with 0.100 M NaOH
CH, COOH(aq) + NAOH(aq) - CH,CO0 (aq) + H.O) + Na*(aq)
Determine the initial pH of the 0.118 M acetic acid solution before NaOH is added.
pH =
Determine the pH of the solution after 10.0 ml of 0.100M NAOH is added.
pH =
Determine the volume of 0.100 M NAOH required to reach halfway to the stoichiometric point of the titration.
volume NaOH:
TOOLS

Transcribed Image Text:+ TOOLS
Determine the ph
NaOH has been added to reach halfway to the stoichiometric point.
pH =
Determine the volume of 0.100 M NaOH that is required to reach the stoichiometric point of the reaction
volume NaOH:
Calculate the pH of the solution when the stoichiometric point of the titration has been reached.
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Dr. Dahm has prepared 250 mL of a acetate buffer which contains 0.1 mol of acetic acid (CH₂COOH) and 0.15 moles of acetate (CH₂COO). Determine the initial pH. If he then adds 0.025 moles of H* calculate the new pH. Ka = 1.8 × 10³ CH₂COOH(aq) + H₂O CH₂COO (aq) + HO (aq) 3. Dr. Dahm makes a second acetate buffer exactly the same way as in #2, but he then adds 15 mL of 0.50 M NaOH calculate the new pH.arrow_forwardRainwater is slightly acidic due to dissolved CO₂. Use the following data to calculate the pH of unpolluted rainwater at 22.6°C: vol % in air of CO₂ = 0.063 vol%; solubility of CO2 in pure water at 22.6°C and 1.09 atm is 79.29 mL CO2 per 100 mL, K₁1 of H₂CO3 = 5.27x10-7. Express the pH with 2 decimals. You might have to solve a quadratic.arrow_forwardThe curve for the titration of 25.0 mL of 0.100 M C5H5N(aq), pyridine, with 0.100 M HCI(aq) is given below. 12 10 8 4 2 5 10 15 20 25 30 35 Va(mL) (If the image above does not appear, click here.) Estimate the pH at the stoichiometric point. Your answer must contain only one decimal place. 8.8arrow_forward
- A volume of 500.0 mL of 0.170 M NaOH is added to 535 mL of 0.250 M weak acid (?a=3.06×10−5). What is the pH of the resulting buffer? HA(aq)+OH−(aq)⟶H2O(l)+A−(aq) pH=arrow_forward(a) Lactic acid, (CH3CH(OH)COOH) is a common biomolecule which can accumulate in muscles during intense exercise. A chemist prepares a buffer using 225 mL of 85 M lactic acid (Ka = 1.38 x 10-4) and 435 mL of 0.68 M sodium lactate. What is the pH of the buffer? What is the pH of the lactate buffer if 0.25 moles of gaseous HCl is added?arrow_forwardA student prepares a solution for titration by adding a 10.00 mL aliquot of a solution saturated with Ca(OH)2 at 82.9°C to 25.0 mL of deionized water. This solution is titrated with a standardized 0.01252 M HCl solution. The equivalence point is observed after 17.94 mL of the HCl solution has been added. Calculate the moles and molarity of OH- and Ca2+ in the 10.00ml sample.arrow_forward
- Calculate the pH for each case in the titration of 50.0 mL of 0.110 M HClO(aq)0.110 M HClO(aq) with 0.110 M KOH(aq).0.110 M KOH(aq). pka = 7.40 ka= 4.0*10^-8 What is the pH after addition of 50.0 mL KOH? What is the pH after addition of 60.0 mL KOH?arrow_forwardCalculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO(aq) with 0.230 M KOH(aq). Use the ionization constant for HCIO. What is the pH before addition of any KOH? pH What is the pH after addition of 25.0 mL KOH? pH = What is the pH after addition of 30.0 mL KOH? = pH = pH = What is the pH after addition of 50.0 mL KOH? pH What is the pH after addition of 60.0 mL KOH?arrow_forwardIn some natural systems the pH must be maintained within very narrow limits. e.g. in human blood the pH must remain close to 7.4 or cell deterioration occurs. Blood contains several weak acid/conjugate base equilibria called buffers, which control the pH.One weak acid present in blood is the dihydrogen phosphate ion, H2PO4-(aq), for which the equilibrium in aqueous solution is H2PO4-(aq) +H2O(l) <--> HPO42-(aq) + H3O+(aq). a) What would be the effect on this equillibrium of adding hydrochloric acid solution?b) Write the expression for the equilibrium constant of this reaction.c) If 0.5 mol H2PO4- and 0.5 mol HPO42- are in equilibrium in 1.0L of aqueous solution, calculate the pH of the solution. (Ka for H2PO4-= 6.4 x 10-8)d) 0.010 mol HCl is now added to the 1.0L of the solution in c). Assuming that all the added H+ ions are used up in the equilibrium shift, calculate the concentrations of H2PO4- and HPO42-. Hence calcuate the pH of this solution.e) 0.010 mol HCl has been added…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY