
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
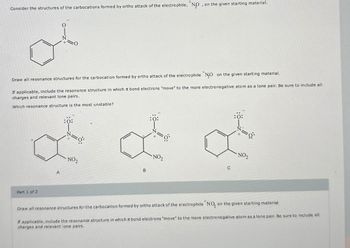
Transcribed Image Text:Consider the structures of the carbocations formed by ortho attack of the electrophile, "NO, on the given starting material.
Draw all resonance structures for the carbocation formed by ortho attack of the electrophile NO on the given starting material.
If applicable, include the resonance structure in which π bond electrons "move" to the more electronegative atom as a lone pair. Be sure to include all
charges and relevant lone pairs.
Which resonance structure is the most unstable?
Part 1 of 2
:0:
NO2
A
B
:0:
:Ö:
а
NO₂
C
NO₂
+
Draw all resonance structures for the carbocation formed by ortho attack of the electrophile "NO, on the given starting material.
If applicable, include the resonance structure in which I bond electrons "move" to the more electronegative atom as a lone pair. Be sure to include all
charges and relevant lone pairs.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 8 images

Knowledge Booster
Similar questions
- A certain hydrocarbon had the molecular formula C16H26 and contained two triple bonds. Ozonolysis gave CH3(CH2), CO₂H and HO₂CCH2CH2CH2CH2CO₂H as the only products. Draw a reasonable structure for this hydrocarbon. Click and drag to start drawing a structure. D:arrow_forwardCH=CHCN 3-phenylpropenenitrile Electrophilic substitution on 3-phenylpropenenitrile occurs at the meta position. Draw resonance structures to show how the ring is electron-poor at the ortho and para positions. ● • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the → symbol from the drop-down menu.arrow_forwardOnly typed answer otherwise leave it not hand writtenarrow_forward
- Complete the equation for the reaction between the following Lewis acid-base pair. Use curved arrows to show the flow of electrons in the reaction and draw the product. Assign lone pairs and radical electrons where appropriate. Apply formal charges where appropriate. • Draw the appropriate electron-flow arrows. • Use the "starting points" menu to revert to the original molecule(s) shown. • Omit + signs between structures. ● / CH3 1- H₂C-C CH3 H در St ? ChemDoodleⓇarrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. PPhs THF • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If the reaction produces a racemic mixture, draw both stereoisomers. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Include anionic counter-ions, e.g., I, in your answer, but draw them in their own sketcher.arrow_forwardPlease do question one, and indicate which resonance form is a greater contributor to the resonance hybridarrow_forward
- What is the missing reactant in this organic reaction? || CH3-C- CH2 -NH2 R No Answer A CH3 CH3-N-CH₂ || C−NH–CH2 C-CH3 + H₂O Specifically, in the drawing area below draw the structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Click and drag to start drawing a structure. || X :0 Śarrow_forwardThe compound below is treated with chlorine in the presence of light. H₂C CH3 H₂C CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Undoarrow_forwardQuestion: Draw a mechanism for the following reaction:arrow_forward
- Draw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. H20/ H,SO, / HgSO4 CH,CH2-CEC-CH, • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu.arrow_forwardOrganic Functional Groups Identifying positions labeled with Greek letters in acids and derivatives If possible, replace an H atom on the a carbon of the molecule in the drawing area with a methyl group substituent, and replace an H atom on the ß carbon with a hydroxyl group substituent. If one of the substituents can't be added for any reason, just don't add it. If neither substituent can be added, check the box under the drawing area. HO Oneither substituent can be added. OH 0/5 Xarrow_forwardDraw both resonance structures of the anion formed by the reaction of the most acidic C-H bond of the compound below with base. Include all valence lone pairs in your answer. For structures having different hydrogens of comparable acidity, assume that the reaction occurs at the less-substituted carbon. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the ↔ symbol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY