
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please solve and read question carefully the answer is not 11977.487.
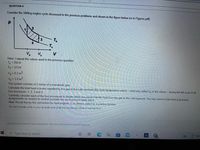
Transcribed Image Text:QUESTION 4
Consider the Stirling engine cycle discussed in the previous problems and shown in the figure below (or in Figures.pdf).
Th
T.
3
V
Here, I repeat the values used in the previous question.
Tc= 293 K
Th = 373 K
Va = 0.2 m3
Vb = 1.0 m3
The system consists of 2 moles of a monatomic gas.
Calculate the total heat in joules expelled by the gas to the cold reservoir (the room temperature water) – what was called Q, in the videos – during the full cycle of all
four processes: 1, 2, 3 and 4.
Carefully consider each of the four processes to decide which processes transfer heat from the gas to the cold reservoir. You may want to look back at previous
assignments (or exams) to remind yourself how each process takes place.
Hint: Recall that by the convention for heat engines Q, is always stated as a positive number.
Do not include units in your answer and write the numerical value in normal form.
Click Save and Subrmit to save and submit. Click Save All Answers to save all ansuers,
Rair
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Show the tolerance zone created by each of the given tolerance specfication. Superimpose the tolerance zone on the given drawing.arrow_forwardQuestion 3 The figures (1&2) shows a Hole-Pattern as drawn and as inspected. fill table 1 and calculate the true position tolerance. Show your calculations. Please note: Dimensions shown is relative to the actual size As drawn 4XØ0500 -0.312 Oe.005(M A BC C Pattern-4 holes # 2 1.000 # 3 +) 1.500 +)arrow_forwardI need help solving problem numbers 4--15.arrow_forward
- I am attaching both questions for 4 and 5 with the question in the image. thank you. NOTE : So the last person answered this question WITHOUT refencing the answer for whether question 4 or 5 answeres were given, so i am asking for question 5(or the answer for the question that was NOT solved because it was not referenced.) These were the following answers given to me from the last person on bartleby who answered my question without referencing whether it was the answer for question 4 or 5. 1 pass 2 fail 3 fail 4 passarrow_forwardI need these three parts answered, if you are unable to answer all three parts please leave it for another tutor to answer, thank you.arrow_forwardPlease Asaparrow_forward
- Please Fill In the blanks where I have marked red X's using the questions and Image below. 1. On the drawing above, before any geometric controls are applied, what is the coaxility tolerance is implied between the diameters? 2. On the part above, apply datum feature symbols to create a datum axis A-B by using the right and left diamters. 3. Apply a position coaxility between the right and left diamter of 0.1 at MMC. 4. Apply a position tolerance to the large center diameter within a diameter of 0.3 in relation to the single datum axis established by both datum features A and B.arrow_forwardU copy from anywhere I need answer only step by steparrow_forwardHow solve do you this 3 part question.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY