
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
4 and 5 please, I’m stuck on these
Transcribed Image Text:9-CHEM 110 Worksheet week
Design
Layout
References
Mailings
Review
View
Help
Insert
Draw
Home
TECTED VIEW Be careful-files from the Intemet can contain viruses. Unless you need to edit, it's safer to stay in Protected View.
C) sp
D) sp
E) sp
Enable Editing
sp
sp
sp
sp
sp
sp
sp
sp
sp
C-1
C-2
C-4
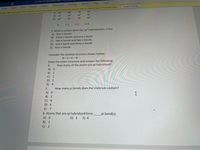
3. When a carbon atom has sp hybridization, it has
A) four n bonds
B) three n bonds and one o bond
C) two n bonds and two o bonds
D) one n bond and three o bonds
E) four o bonds
Consider the skeletal structure shown below:
N-C-C-N
Draw the Lewis structure and answer the following:
4.
How many of the atoms are sp hybridized?
A) 0
B) 1
C) 2
D) 3
E) 4
5.
How many pi bonds does the molecule contain?
A) 0
B) 2
C) 4
D) 6
E) 7
6. Atoms that are sp hybridized form
A) 0
B) 1
C) 2
pi bond(s).
D) 3
E) 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You dissolve 14 g of Mg(NO,2 in water and dilute to 750 mL. What is the molarity of this solution? Show your work.arrow_forward2. Explain how suction filtration is carried out in the laboratory. Give the important points that must be observed in doing the process.arrow_forwardcan i get help, i thought this was the answer and its notarrow_forward
- Can someone help with this multistep sytheisis and explain why they got their answers.arrow_forward7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forwardAnswer 3 and 4arrow_forward
- Figure 11-5 A. 1) Hg(0Ac)₂, H₂O B. 1) BH 3 C. D. 2) Na B H4 2) H₂O₂, OH, H₂O 2) OH, DMF ) 1) H Br 1) Hg (0Ac) ₂, H₂O H Br → 3) HBr 3) Ts Cl 2) Brz 3) TSCI ) Br H₂ SO4 4) Br¯ 4) HBrarrow_forwardBalance each of the following neutralization reactions. Part A HNO, (aq) + Sr(OH)2(s)→H20(1) + Sr(NOs)2(aq) Express your answer as a chemical equation. Identify all of the phases In your answer. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Request Answer t Speec.pdfarrow_forwardFor problems 1-4 you are not expected to come up with an exact structure. Use the provided data to identify functional groups the molecule contains and propose some possible structures that fit the dataarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY