
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
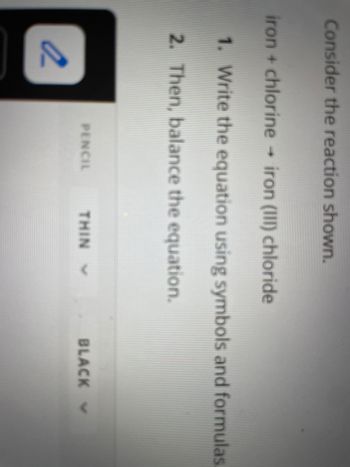
Transcribed Image Text:Consider the reaction shown.
iron + chlorine → iron (III) chloride
1. Write the equation using symbols and formulas.
2. Then, balance the equation.
2
PENCIL
THIN
BLACK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mary is a lab assistant. She has 200.0 mL of a 1.75 M cupric chloride solution. She is going to remove the copper using solid aluminum in a single replacement reaction. What mass of aluminum will she need to remove all the copper? You will need a balanced equation to solve thisarrow_forwardA pair of students in chemistry lab are performing chemical reactions lab. They mix zinc nitrate with sodium carbonate. The reaction produces a precipitate in the bottom of their test tube. The lab manual states, "All common nitrates (NO3) are soluble. All carbonates (CO3)2- are insoluble except with group 1 or NH4* ions." Select the chemical reaction that shows the correct, balanced chemical reaction between zinc nitrate and sodium carbonate. Zn(NO3)2 (aq) + N22CO3 (aq) → No reaction Zn(NO3)2 (aq) + N22CO3 (aq) → ZnNa2 (s) + CO3(NO3)2 (aq) Zn(NO3)2 (aq) + Na2CO3 (aq) → ZNCO3 (s) + 2 NaNO3 (aq) Zn(NO3)2 (aq) + Na2CO3 (aq) -→ ZNCO3 (aq) + 2 NANO3 (s)arrow_forwardBalance the following reaction _____ C8H18 + _____O2 -------> _____CO2 + _____ H2Oarrow_forward
- The following is the formula for which of the following reactions? Combination or Synthesis Reaction Decomposition Reaction Single Replacement Reaction Double Replacement Reaction Combustion Reaction acer #3 $4 % & 3 4 6. 7 y 00arrow_forwardBalance the following reaction _____ C5H10O + _____O2 -------> _____CO2 + _____ H2Oarrow_forward5570/assessmentS/ 271550/ SHORT ANSWER Question 2 In a balanced equation, the same number of each kind of atom is shown on each side of the equation. Calculate the number of iron (Fe), oxygen (O), and carbon atoms (C) in the equation. Fe,0, +3CO 2Fe + 3CO, Reactants iron oxygen carbon Products iron oxygen carbon Based on these values, is the equation balanced? Answer in a complete sentence.arrow_forward
- Balance the following reactions _____ C6H6 + _____ O2 ---> _____ CO2 + _____ H2Oarrow_forwardsodium bromide + calcium nitrate a)What is the molecular equation? b) What is the total ionic equation? c) What is the net ionic equation?arrow_forwardComplete and balance the following reaction, then give the product side of the balanced equation. __ Cu (s) + __ ZnSO4 →arrow_forward
- balance this chemical reaction ____ NaHCO3 + ____ C6H8O7 → ____ Na3C6H5O7 + ____ H2O + ____ CO2arrow_forwardComplete and balance the following reaction, then give the product side of the balanced equation. __ Cu (s) + __ ZnSO4 →arrow_forwardWhich of the following is the correct initial atom inventory for the chemical reaction below? (You do not need to balance)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY