
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:[o
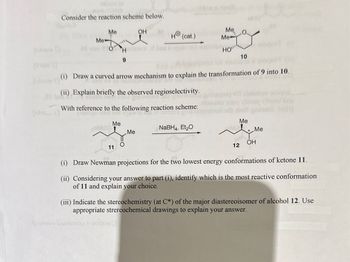
Consider the reaction scheme below.
Me
Me
of 210
210
COMI20
OH
Hola
Holensal,d bus
9
(ii) Explain briefly the observed regioselectivity.
Iviz gniw
11
toshsvo beiniinoo noitz9
H® (cat.)
With reference to the following reaction scheme:
[monqo 1250 901 ayawis luna no
br
Me
NaBH4, Et₂O
(i) Draw a curved arrow mechanism to explain the transformation of 9 into 10.
of main
Me
101 anoitiba Ho
HO
Me, O
Me
10
100 10 Stone &
arq tot abortom saogo
atowens 1004 Viitzut vford bre
ooort mott gnis2 H)
osogor (1)
Me
Miel Me
12
*
OH
(i) Draw Newman projections for the two lowest energy conformations of ketone 11.
(ii) Considering your answer to part (i), identify which is the most reactive conformation
of 11 and explain your choice.
(iii) Indicate the stereochemistry (at C*) of the major diastereoisomer of alcohol 12. Use
appropriate strereochemical drawings to explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- pt pt pt 1 pt C|Chec C Chee C I need 8 https://east.cengagenow.com/ilrn/takeAssignment/take CovalentActivity.do?locator-assignment-take b Answe F3 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. [References] Draw the structure of the one tertiary (30) alcohol with the molecular formula C5H120. Submit Answer F4 90-85 4+ F5 ¤- CHA 95 IF6 C Draw *+ a O. IF F7 C/C MOTION EYE C Draw F8 SONY Draw F9 C Home WEB k ak DISPLAY F10 F11 C-Draw C One Touch Web Access Withcarrow_forwardLl.43.arrow_forwardN acin OH ACS ACS 19. Which indicated nitrogen atom is most basic? (A) (C) (D) CH3 20. What is the lowest-unoccupied molecular orbital (LUMO) of 1,3-butadiene? (A) (C) (A) O : Br: H₂C H₂O: (B) 21. What is a mechanistic step for this reaction? Br₂ H₂O (D) CH3 N (B) (B) N-(C) (D) H₂O: OH :Br:arrow_forward
- Give the major product of the following reaction.. ol NN excess (1) CH₂CH₂CH₂ A" H Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template DCQ, H 12DE CONT ? 1. OH, HO 2. A. -H₂0 d Marvin JS by ChemAxon H C N O S CI Br 1 P Farrow_forwardDraw the chemical structure of the following organic compounds and identify each one as an alkane, an alkene, or an alkyne: a. C4H6 b. C3H8 c. C6H12 « Previousarrow_forwardPredict the product in the following reactions: Br H3C mation CF3 ساله Br F DBU OH conc. H₂SO4 1 equiv. NaN3 DMSO Coor HCIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY