
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show all work, thank you!
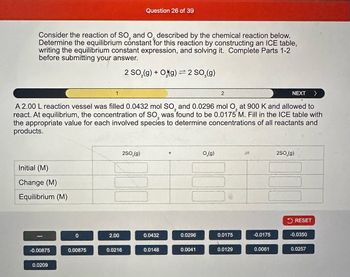
Transcribed Image Text:Consider the reaction of SO₂ and O described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 SO₂(g) + Og) = 2 SO₂(g)
NEXT >
A 2.00 L reaction vessel was filled 0.0432 mol SO₂ and 0.0296 mol O₂ at 900 K and allowed to
react. At equilibrium, the concentration of SO, was found to be 0.0175 M. Fill in the ICE table with
the appropriate value for each involved species to determine concentrations of all reactants and
products.
Initial (M)
Change (M)
Equilibrium (M)
-0.00875
0.0209
0
0.00875
2.00
Question 26 of 39
0.0216
2SO₂(g)
0.0432
0.0148
0.0296
0.0041
2
0₂(g)
0.0175
0.0129
-0.0175
0.0061
2SO₂(g)
RESET
-0.0350
0.0257
![Consider the reaction of SO, and O, described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 SO₂(g) + O₂(g) 2 SO₂(g)
PREV
[0.0432]
Based on the set up of your ICE table, construct the expression for Kc. Each reaction participant
must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kc.
[0.0296]²
2.99 × 10³
[0.0296]
[0.0175]²
3.35 x 10
Question 26 of 39
Кс =
[0.0175]
[0.0041]²
[0.0041]
[0.0129]²
[0.0129]
2
[0.0061]²
[0.0061]
[0.0257]²
[0.0257]
[0.0209]²
RESET
[0.0209]
7.00 x 10²](https://content.bartleby.com/qna-images/question/627ffe10-a730-4e4c-b7e9-b306478e5985/e0c0b2e9-c5f6-433c-b057-78acc95199e5/lj1r86h_thumbnail.jpeg)
Transcribed Image Text:Consider the reaction of SO, and O, described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 SO₂(g) + O₂(g) 2 SO₂(g)
PREV
[0.0432]
Based on the set up of your ICE table, construct the expression for Kc. Each reaction participant
must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kc.
[0.0296]²
2.99 × 10³
[0.0296]
[0.0175]²
3.35 x 10
Question 26 of 39
Кс =
[0.0175]
[0.0041]²
[0.0041]
[0.0129]²
[0.0129]
2
[0.0061]²
[0.0061]
[0.0257]²
[0.0257]
[0.0209]²
RESET
[0.0209]
7.00 x 10²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forwardUsing the proper conversion factor, convert 8.4 ft? into cm2. ! cm? Save for Later Last saved 1 day ago. Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes. Part 3 The parts of this question must be completed in order. This part will be available Part 4 Te ortc of thic question must he comnleted in order This part will be availablearrow_forwardAll changes save 7. When two solutions are mixed, a color change occurs. The data tables show the time between mixing and the color change for two sets of conditions. For Condition One, the solution concentrations were constant and temperature varied. For Condition Two, the temperature was constant and concentrations varied. Condition One: Concentration Time for Temperature (°C) Sample Color to Change 1 10° 36 sec 22° 14 sec Condition Two: Temperature Time for Concentration Sample Color to % Change 1. 100% 15 sec 2. 50% 24 sec Which of these statements is true according to the data? O The reaction rate is greater at 22°C than at 10°C. O Reducing the temperature increases the rate of the reaction. The reaction is affected by changes in temperature not by changes in concentration. O Decreasing the concentration increases the reaction rate. PREVIOUS 17 of 25 NEXT SAVE & EXITarrow_forward
- 10,11arrow_forwardAutoSave We hw OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo.. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe BIU V ab A • I v A v Paste х, х E= == No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity C13 NMR Peaks Aldehydes RCO)R Aldehydes and ketones Carbaxylic R(CO)X Carbaxylic acid derivatives Ntrile Nitrile RCN CC C-C Alkyne Akyne R-CC-R RCH20 RCH2-O R4C RAC R3CH R3CH RCH2X X= C-C, C-O, Br, CI, N RCH2X R2CH2 RECH2 RCH3 RCH3 TMS TMS 220 200 180 160 140 120 100 80 60 40 20 Typical chemical shifts in 13c-NMR 9) Why do the peaks of associated with aldehydes (both in H NMR and C NMR) appear so much further downfield than other peaks? C13 NMR Peaks E Page 5 of 5 E English (United States) O Focus 295 words 白arrow_forwardHi, can you answer part A with a very detailed answer please? Thank you!arrow_forward
- Using chemicals to restore obliterated identifiers on firearms, tools, vehicles, boats, etc. is known as: Group of answer choices Serial Number Restoration Restorative Analysis Identification Tool Mark Recoveryarrow_forwardParaphrasing Tool | QuillBot AI X b Answered: Part A Classify each x Course Home Dashboard UC 6 Yoga Asanas To Help You Bur X openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=6acab9256cb89b6deOb7314be966bc5a#10001 Apps Yahoo Mail YouTube Maps Best Free PowerP... Google Drive on Academic Search Downloads € University Librarie... E UNIVERSITY POR... Student Detail Sc... > I Review I Constants I Periodic Table Scores Balance each of the following by determining coefficients, and identify the type of reaction. eТext Part A Document Sharing Identify the coefficients in the reaction: User Settings Course Tools > Enter your answers in order from left to right numerically separated by commas. Use the lowest possible coefficients. ? Submit Request Answer Part B Identify the type of reaction in Part A. combination double replacement combustion decomposition P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions |…arrow_forwardPlease Describe and specify the use made of the equipment shown.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY