
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
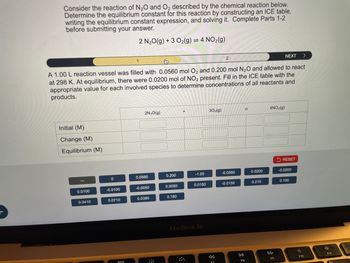
Transcribed Image Text:Consider the reaction of N2O and O2 described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 N2O(g) +3 O2(g) = 4 NO2(g)
SH
2
NEXT >
A 1.00 L reaction vessel was filled with 0.0560 mol O2 and 0.200 mol N2O and allowed to react
at 298 K. At equilibrium, there were 0.0200 mol of NO2 present. Fill in the ICE table with the
appropriate value for each involved species to determine concentrations of all reactants and
products.
Initial (M)
Change (M)
Equilibrium (M)
2N₂O(g)
302(g)
==
4NO2(g)
RESET
0
0.0560
0.200
-1.00
-0.0560
0.0200
-0.0200
0.0100
-0.0100
-0.0050
0.0050
0.0150
-0.0150
0.210
0.190
0.0410
0.0710
0.0360
0.180
OOD
MacBook Air
ུཥ
AA
DII
DD
F8
F9
D
F10
F11
E
![Consider the reaction of N2O and O2 described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 N2O(g) +3 O2(g) = 4 NO2(g)
<
PREV
1
2
Based on the data from your ICE table, construct the expression for Kc. Each reaction participant
must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kc.
Kc
=
RESET
[0.200]
[0.0560]
[0.0200]
[0.190]
[0.0410]
[0.0710]
[0.0360]
[0.180]
[0.200]²
[0.0560]³
[0.0200]⭑
[0.190]²
[0.0410]³
[0.0710]³
[0.0360]³
[0.180]
0.0643
2.57
15.6
0.390
MacBook Air](https://content.bartleby.com/qna-images/question/42021111-4ced-4c6f-84e9-5b1969157ec2/45eab6d3-590f-437f-9efa-9a1d58a24370/ej2cqrc_thumbnail.jpeg)
Transcribed Image Text:Consider the reaction of N2O and O2 described by the chemical reaction below.
Determine the equilibrium constant for this reaction by constructing an ICE table,
writing the equilibrium constant expression, and solving it. Complete Parts 1-2
before submitting your answer.
2 N2O(g) +3 O2(g) = 4 NO2(g)
<
PREV
1
2
Based on the data from your ICE table, construct the expression for Kc. Each reaction participant
must be represented by one tile. Do not combine terms.
Once the expression is constructed, solve for Kc.
Kc
=
RESET
[0.200]
[0.0560]
[0.0200]
[0.190]
[0.0410]
[0.0710]
[0.0360]
[0.180]
[0.200]²
[0.0560]³
[0.0200]⭑
[0.190]²
[0.0410]³
[0.0710]³
[0.0360]³
[0.180]
0.0643
2.57
15.6
0.390
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating equilibrium composition from an equilibrium constant Suppose a 250, mil. flask is filled with 1.5 mol of NO₂, 0.10 mol of CO and 2.0 mol of CO₂. The following reaction becomes possible: NO₂(g) +CO(g) NO(g) + CO₂(g) The equilibrium constant K for this reaction is 0.226 at the temperature of the flask. Calculate the equilibrium molarity of NO₂. Round your answer to two decimal places. OM Explanation CIU Check DEC 9 X 3 6 tv 8arrow_forwardFor the reaction H₂(g) + CO₂(g) → H₂O(g) + CO(g) at 700 °C, K=0.534. Calculate the number of moles of H₂ that are present at equilibrium if a mixture of 0.660 mole of CO and 0.660 mole of H₂O is heated to 700 °C in a 30.0 L container. Round your answer to 3 significant digits. mol x10 Xarrow_forwardPlease don't provide handwritten solution.....arrow_forward
- 2arrow_forwardO KINETICS AND EQUILIBRIUM Calculating equilibrium composition from an equilibrium constant Suppose a 500. mL flask is filled with 2.0 mol of NO, and 1.3 mol of NO. The following reaction becomes possible: NO, (g) + NO(g) → 2NO, (g) The equilibrium constant K for this reaction is 6.91 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places. Explanation Check 02022 McGraw Hill LLC. All Rights Reserved. Terms of Use |P 21arrow_forwardSuppose a 500.mL flask is filled with 2.0mol of CO, 0.80mol of NO and 0.70 mol of CO2. The following reaction becomes possible: NO2g + COg ⇌ NOg + CO2g The equilibrium constant K for this reaction is 0.337 at the temperature of the flask. Calculate the equilibrium molarity of NO2. Round your answer to two decimal places.arrow_forward
- A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: N₂(g) + 2H₂O(g) 2NO(g) + 2H₂(g) K₂=2. × 108 He fills a reaction vessel at this temperature with 14. atm of nitrogen gas and 9.4 atm of water vapor. Use this data to answer the questions in the table below. Can you predict the equilibrium pressure of H₂, using only the tools yes available to you within ALEKS? If you said yes, then enter the equilibrium pressure of H, at right. Round your answer to 1 significant digit. no 03 atm X Sarrow_forwardA mixture of 0.10 mole NO, 0.050 mole H₂, and 0.10 mole H₂O is placed in a 2.0 L vessel. The following equilibrium is established: + 2 H₂ (g) = N2(g) + 2 H₂O (g) 2 NO At equilibrium [NO] = 0.030 M. The initial concentration of N₂ was not mentioned so you must assume [N₂] = 0. Calculate the equilibrium concentrations (in M) of: More Information X H₂ (g) XN₂ (g) X H₂O (g) Question Answer Your answer -.020 is Your answer .035 is in Your answer .170 is i Tol: 0.0001 Tol: 0.001 Tol: 0.001arrow_forwardConsider the equilibrium system described by the chemical reaction below. For this reaction, Kc = 2.4 x 103 at a particular temperature. If the equilibrium concentrations of H,0 and H, are 0.11 M and 0.019 M, respectively, determine the concentration of O, at equilibrium.. 2 2 H,O(g) = 2 H,(g) + 0,(g) 2 NEXT > If [x] represents the equilibrium concentration of O, set up the equilibrium expression for Kc to solve for the concentration. Do not combine or simplify terms.. 2' K. = = 2.4 x 103 5 RESET [О.11] [0.019] 2[0.11] 2[0.019] [0.11]? [0.019]? [x] [x]? [2x] [2x]?arrow_forward
- Nitrosyl bromide decomposes according to the equation given below. If 0.640 mole of NOBr is placed in a 1.00 L flask containing no NO or Br2 it is found that at equilibrium there is 0.460 mole of NOBr. What is the value of Kc for this reaction? 2 NOBr (g) → 2 NO (g) + Br2 (g) 1.38 x 10−2 7.26 7.12 x 10−3 3.52 x 10−2 28.4arrow_forwardSuppose a 250. mL flask is filled with 0.70 mol of NO₂, 0.20 mol of NO and 1.9 mol of CO₂. The following reaction becomes possible: NO₂ (g) + CO (g) NO(g) + CO₂(g) The equilibrium constant K for this reaction is 0.715 at the temperature of the flask. Calculate the equilibrium molarity of NO₂. Round your answer to two decimal places.arrow_forwardWrite the pressure equilibrium constant expression for this reaction. 2CH3OH(l)+3Os(g) --> 2CO2(g)+4H2O(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY