
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
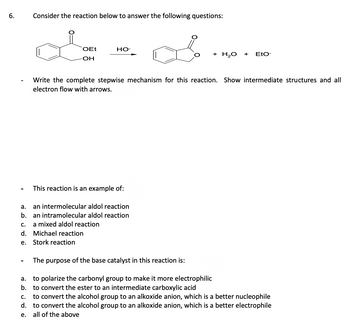
Transcribed Image Text:6.
Consider the reaction below to answer the following questions:
OEt
-OH
HO-
This reaction is an example of:
a.
an intermolecular aldol reaction
b. an intramolecular aldol reaction
a mixed aldol reaction
C.
d. Michael reaction
e.
Stork reaction
+ H₂O
Write the complete stepwise mechanism for this reaction. Show intermediate structures and all
electron flow with arrows.
+
EtO-
The purpose of the base catalyst in this reaction is:
a. to polarize the carbonyl group to make it more electrophilic
b. to convert the ester to an intermediate carboxylic acid
C.
to convert the alcohol group to an alkoxide anion, which is a better nucleophile
d. to convert the alcohol group to an alkoxide anion, which is a better electrophile
e. all of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20. Provide the reactants and reaction conditions necessary for the following transformations. Stereochemistry and regiochemistry may be important. a. b. C. d. type: Oxidative cleavage type: Fisher esterification type: Acetal formation type: Aldol condensation TSOH OH + CO2 + H2O & volonarrow_forwardPlease don't provide handwriting solutionarrow_forwardMelphalan, a drug used in chemotherapy, reacts with itself in the body before binding with its target, as illustrated in the mechanism below. What is the first pattern of arrow pushing seen in this reaction? (Look at the first arrow coming from the Nitrogen with a lone pair). a. rearrangementc. nucleophilic attackarrow_forward
- A 1o, symmetrical epoxide undergoes a ring-opening addition reaction when treated with ammonia (NH3). Mechanistically, what is the first step? Assume base/neutral conditions. Question 9Answer A. The C-C bond of the epoxide ring breaks. B. NH3 nucleophilically attacks the epoxide carbon, breaking the C-O bond. C. NH3 attaches to the epoxide oxygen. D. The epoxide oxygen is protonated by NH3 .arrow_forwardWhy is the reaction of the type shown below usually done? a.To make an aldehyde or ketone less water soluble b.To make the molecule more reactive c.To protect a ketone or aldehyde carbonyl d.To increase the oxygen content e.To make the alpha hydrogens more acidicarrow_forwardPlease show reaekson and don't use hend raitingarrow_forward
- 9. Explain the reaction shown below. Include the following pieces in your explanation. Identify the electrophile and the nucleophile. a. b. C. Draw a curved arrow mechanism for this reaction. (Assume an acidic work-up). Explain the electron flow. d. Indicate any regiochemical or stereochemical preferences. 1) NO 0 2) HCI, H₂O NH2 CIarrow_forward13 saved out of on 14 r saved out of Given the following substitution reaction, what would the effect be of changing the solvent from DMF to CH₂OH? (see NOTE) *NOTE: DMF - NaOCH3 H-C-N(CH3)2 OCH 3 Select one: OA. There would be no effect on the reaction rate. OB. The rate of the reaction would increase. OC. The rate of the reaction would decrease. Clear my choice + Na Br In substitution reactions, (CH3)3C-I reacts at the same rate with Brand CI even though Br is a more reactive nucleophile than Cl. Why? maxarrow_forward4. In the following compound: (a). Circle the good leaving group. (b). Explain why it is a good leaving group. HO,arrow_forward
- A. Write appropriate reagents over the reaction arrow B. As a result of this reaction, is the N made more nucleophilic or less nucleophilic?arrow_forward15.Which of the following correctly explains why alkyl groups are ortho/para-directing groups? a. Meta substitution results in an arenium ion in which a positive charge is destabilized by an adjacent electron-withdrawing group. b. Ortho/para substitution results in an arenium ion intermediale in which all atoms have complete octets. c. A lone pair of electrons on the alkyl group participates in re sonance and stabilizes the arenium ion intermediate in ortho/para substitution. d. The most stable resonance structure for the arenium ion intermediate in ortho/para substitution contains a 2° carbocation. e. The alkyl group is electron donating via induction and stabilizes the adjacent positive charge in the ortho/para substitution intermediate. 5.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY